
pharmacologically regulated cell therapy
We are developing a new generation of cell therapies that involves placing cells under the "remote control" of small molecule drugs. Over the past decade we have completed much of the ground-work establishing the feasibility of this approach. We believe that the approaches we are developing today will be commonplace 100 years from now (see the last chapter of "Thomas' Hematopoietic Cell Transplantation," written by Ernie Beutler), but we are most interested in pursuing approaches with (hopefully) nearer-term clinical applications.
Off Target Effects Constrain the Clinical Applicability of Growth Factors. The safety and efficacy of a drug are determined by two forms of specificity: specificity of the drug for its target (usually a protein), and specificity of the drug target in the pathogenesis of the disease being treated. Side effects arise when either type of specificity falls short of physiologically dictated thresholds, phenomena collectively referred to as "off-target" effects. Although off-target effects plague all drug development, their impact on the clinical use of hematopoietic growth factors provide a case-in-point, and a little explored rationale for genetic manipulation.
The major hematopoietic growth factors in clinical use today, erythropoietin (Epo), granulocyte colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) were all approved by the US Food and Drug Administration (FDA) more than 15 years ago. Since then no growth factors with novel biological activity have gained widespread use, a situation almost entirely attributable to off-target effects. For example, none of the many potential clinical uses for recombinant stem cell factor (SCF) were realized because its receptor (c-kit) is expressed not only in primitive hematopoietic progenitors, but also in mast cells, causing significant allergic reactions in 10 - 20% of patients. Fibroblast growth factors (FGFs) further illustrate the problem. 23 different FGFs bind 7 different receptor isoforms that are variably expressed in all tissues. Most FGFs activate more than one type of FGF receptor, leading to one type of off target effect. Furthermore, even when an FGF specific for a given receptor is used, the receptor is invariably expressed in multiple cell types, producing a second type of off target effect. Thus clinical trials of FGFs have yielded disappointing results. Over the past decade we have been developing a system that has the potential to circumvent off-target effects.
Pharmacologically Regulated Cell Therapy. An important obstacle to cell therapy is the loss of control over cells that have been transplanted. We used previously described technology to develop a way to regulate the proliferation of engineered cells using a growth factor receptor modified to substitute its normal ligand-binding site with the binding site for a drug called a chemical inducer of dimerization (CID). The CID brings together two copies of the artificial receptor, triggering its activation and leading to cell proliferation, thereby mimicking the effect of a growth factor.
Regulated Cell Therapy
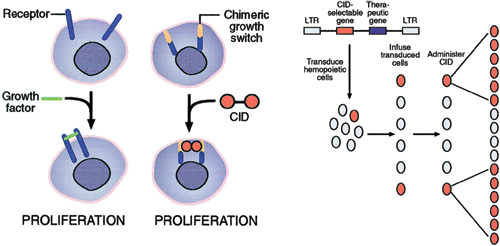
Figure 1. Left panel A proliferation switch consisting of a receptor and a dimerization domain is activated upon addition of a CID, thereby mimicking the effect of growth factors. Right panel: In vivo selection of genetically modified cells using CIDs. The vector may encode a therapeutic gene in addition to the CID selectable gene. Following infusion of transduced cells, the CID specifically induces genetically modified cells to proliferate.
First generation CIDs capitalized on the interaction between a drug, FK506, and its naturally occurring intracellular target, FKBP12, and dimerization was induced by covalently linking two copies of FK506 to generate a new ligand called FK1012. To dramatically reduce undesired interactions between FK1012 and endogenous FKBP12, second generation CIDs were developed using a "bump and hole" approach. These new CIDs (such as AP1903 and AP20187) contain chemical modifications ("bumps") that preclude binding to endogenous FKBP12, but which can be accommodated by a modification of FKBP12 to introduce a pocket ("hole") via a single amino acid substitution (F36V). AP1903 was well tolerated in a Phase I study in normal human volunteers. This approach has a number of advantages, many of which are described in greater detail below. One advantage is its generalizability. A single drug can be used to regulate hundreds of different signaling molecules, to elicit a variety of cellular responses. In previous studies we have used signaling domains taken from the erythropoietin receptor, GCSF receptor, gp130, flt-3, c-kit, Mpl, fibroblast growth factor receptor-1 (FGFR-1), and the 4 Janus kinase family members to induce CID-dependent proliferation. A second advantage is its versatility, allowing for regulated proliferation both in vitro and in vivo. A third advantage is its anticipated lack of immunogenicity, because CID responsive proteins can be entirely human in origin, in contrast to virtually all other regulated systems that rely on the expression of foreign proteins, and are therefore susceptible to immune responses. A fourth advantage is its wide applicability, with examples of CID-regulated proliferation ranging from myoblasts, hepatocytes and pancreatic islet cells, to primary hematopoietic cells, suggesting that CID-regulated approaches might provide an operating system for cell therapy. Hematopoietic applications enjoy a fifth advantage, pleiotropy, because different growth factor signaling domains can elicit different hematopoietic responses, allowing CIDs to regulate hematopoiesis in ways unachievable with conventional growth factors. A sixth advantage of this approach is its specificity. In contrast to conventional growth factors which can bind off-target receptors, or receptors expressed by off-target cell types, CIDs can deliver specific signals to specific cell types. It is this feature that is most relevant for the work described below. We are applying CID regulation to a recently recognized problem related to off-target effects of Epo in patients with cancer related anemia (LINK).
A Platform for Cell Therapy. Preclinical studies have shown that CIDs can be used to regulate the proliferation of pancreatic islet cells for the treatment of diabetes, muscle cells for the treatment of heart failure, and hepatocytes for the treatment of liver disease. A derivative of this approach can be used to bring apoptotic proteins under pharmacological control, allowing for the CID induced killing of genetically modified cells. Thus CID regulation of various signaling molecules can provide a general operating system for cell therapy (Miller & Blau, Gene Therapy 2008).
Recent Review (not Blau Lab): Fegan A, White B, Carlson JCT, Wagner CR Chemically Controlled Protein Assembly: Techniques and Applications Chemical Reviews 110:3315-3336, 2010.