
Prof. Spiro |
|
| |
We are interested in how proteins tune the reactivity of metal ions for ligand binding and catalysis, and how metal ions influence the structure and dynamics of proteins. We use vibrational spectroscopy, Raman and infrared, to address these issues. The vibrational spectra are rich in structural and dynamical information, and modern techniques permit the monitoring of discrete structural units in proteins with high sensitivity and selectivity. In particular, the resonance Raman (RR) effect provides a way to amplify the vibrational bands of a chromophoric unit by tuning the laser to the wavelength of an electronic transition. Excitation in the visible region permits the study of many metal centers, while excitation in the ultraviolet region permits the examination of the aromatic residues of the protein, and of the peptide bonds. Protein Dynamics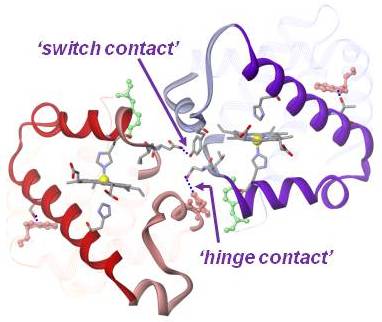
The motions of proteins are of great scientific interest, but are difficult to study directly. Time-resolved Raman spectroscopy holds great promise in this area, because the spectra are sensitive to structure, and because they can now be measured on time scales as short as picoseconds. Tuning of the laser excitations permit to monitor selectivity the resonance Raman [RR] spectra of the heme group [visible region], of tyrosine and tryptophan sidechains [UV region] and backbone amide bonds (deep UV region) in proteins. Because of the sensitivity of amide bands, Raman spectra from amide vibrations are used to monitor the secondary structures in proteins. RR spectra of aromatic residues are sensitive to hydrogen bonding and therefore exploited as a probe of tertiary and quaternary structures in protein. Our protein dynamics research is organized around three different levels of protein motions: those associated with the folding and unfolding of the polypeptide chain, those associated with function (e.g. enzyme catalysis or ion transport) and those associated with regulation of function, i.e. allostery. Laser induced temperature jump/Raman spectroscopy with wavelength tuning into the UV region provides multiple structural probes to monitor the folding and unfolding dynamics in polypeptides and proteins. Protein motions responsible for the allosteric transition in hemoglobin are studied with photo-triggered resonance Raman spectroscopy, combined with site-mutagenesis and hybrid tetramer construction, supported by QM/MM computation. Computational Modeling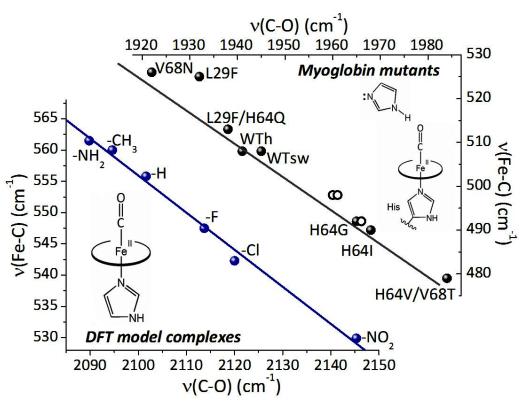
Ab initio electronic structure calculations, especially Density Functional Theory, have advanced to the point where vibrational spectra, as well as structures, can be quite accurately predicted for molecules and metal complexes. We are using these methods to model RR spectra of metalloprotein active sites, in order to evaluate protein influences on the metal center. Photocatalytic Hydrogen Production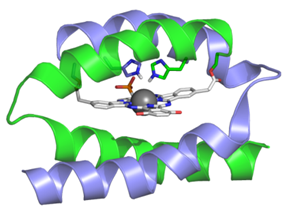
We are collaborating with UW’s C. Luscombe (Materials Science and Engineering) and D. Baker (Biochemistry) in creating metallo-macrocycle-protein constructs as novel photocatalysts for H2 production from water. Our strategy is to develop metal complexes that efficiently absorb solar photons, and then channel the energy into the separation of charge that eventually produces metal ion in its lower oxidation state. In this state, the photo-reduced metal will be capable of generating hydrogen from water, provided that chemical groups are available to transfer hydrogen ions from the water to the metal. To ensure that, we plan to design and synthesize small protein scaffolds that would bind the photocatalyst, and deliver protons to the photo‐reduced metal center and stabilize transition states of the catalytic reaction. 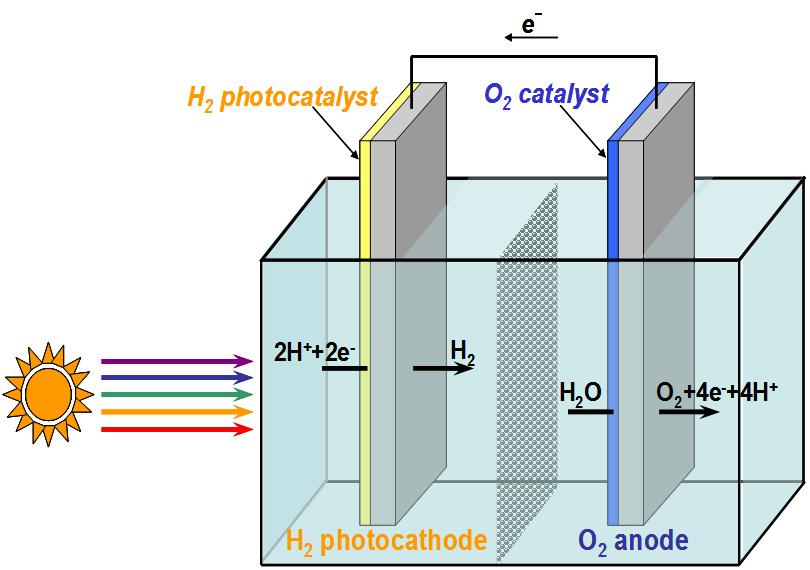
When attached to a p-type semiconductor, the photocatalyst will form half of a photo-electrochemical cell for splitting water. The water‐splitting photocell will be completed by connecting the hydrogen‐generating photo‐electrode to another electrode, which is capable of generating oxygen. Environmental Chemistry
Our research in this area, in collaboration with scientists at Scripps (B.Tebo), UC Berkeley (G.Sposito) and SSRL (J.Barger), is aimed at addressing the bioinorganic and environmental chemistry of manganese oxides. Studies of the surface structure and degradation capabilities of manganese oxides are being explored using a combination of spectroscopic and chromatographic techniques. The biochemical mechanism of manganese oxide formation is being explored.
|
