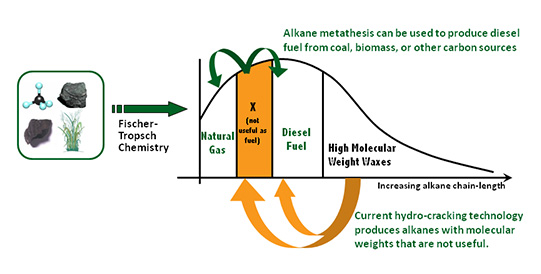
The ability to convert low-molecular-weight alkanes to high-molecular-weight alkanes yields additional sources of transportation fuel such as diesel. Lighter n-alkanes can be obtained via Fischer-Tropsch chemistry from syngas, possibly originating from the current surplus of natural gas in the US, from direct biomass reduction, or even from CO² reduced with the use of sustainable energy sources. Moreover, light alkanes are found in vast amounts in natural gas and petroleum reserves, equivalent to >10% of current world oil reserves.
In 2006 we reported a system for alkane metathesis based on tandem transfer-hydrogenation and olefin metathesis.¹ Since then we have made progress on and continue to move toward a commercially viable alkane metathesis system by improving catalyst selectivity, stability, and rates, as well as demonstrating robust heterogeneous systems. The key component reactions, alkane dehydrogenation and olefin metathesis, are also of great value for many other chemical transformations.
Recent Publications and Patents
© 2007 - 2026 Center for Enabling New Technologies Through Catalysis
centcweb@u.washington.edu
This work was supported by NSF under the CCI Center for Enabling New Technologies Through Catalysis, CHE-1205189. Any opinions, findings, and conclusions or recommendations expressed here are those of the authors and do not necessarily reflect the views of the National Science Foundation (NSF).