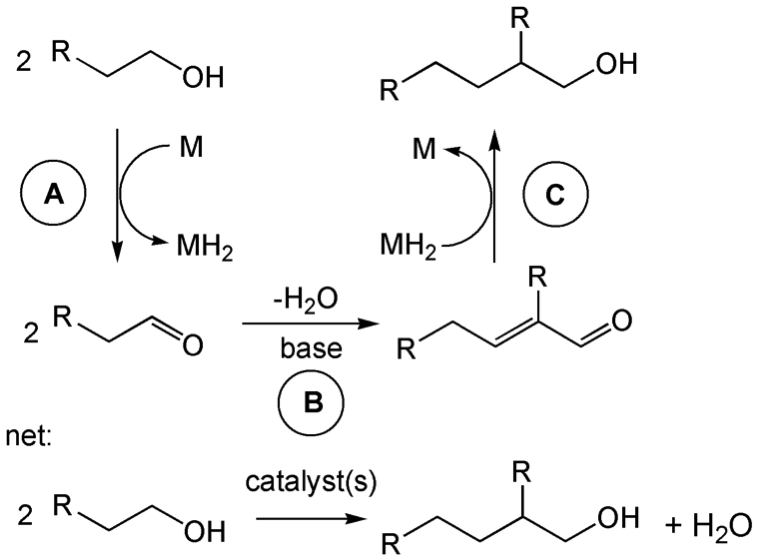
n-Butanol has several advantages over ethanol as a fuel, but the bulk synthesis of n-butanol from sustainable sources is challenging mainly due to poor selectivity issues. We have been developing tandem catalytic routes for the conversion of ethanol to n-butanol via the Guerbet reaction. In the Guerbet reaction, dehydrogenation of ethanol first produces acetaldehyde. Next, base is used to effect an Aldol condensation, and then the dihydrogen produced in the first step is used to hydrogenate the aldol product to produce butanol. We have shown that a combination of iridium and "bulky" nickel/copper catalysts can carry out this reaction with very high selectivity and conversion of ethanol, and are working to improve the process by examining the reactivity of other non-precious metal materials as catalysts.
Recent Publications and Patents
© 2007 - 2026 Center for Enabling New Technologies Through Catalysis
centcweb@u.washington.edu
This work was supported by NSF under the CCI Center for Enabling New Technologies Through Catalysis, CHE-1205189. Any opinions, findings, and conclusions or recommendations expressed here are those of the authors and do not necessarily reflect the views of the National Science Foundation (NSF).