Bio Probes
1. Oxygen and pH Probes
Optically excited emissions (e.g. fluorescence and phosphorescence) of molecular probes are powerful reporters of local environmental parameters such as pH and oxygen partial pressure. We have developed series of “turn on” type molecular probes that can be used to measure the real time intracellular pH of a single live cell via fluorescence imaging. The intracellular pH is sensitively determined by increased fluorescence intensities of the probes due to interrupted electron transfer of excited states of probes followed by protonation of the probes in the cells. Porphyrin derivatives with heavy metal ions are known to undergo efficient inter-system crossing to triplet states when excited. The excited triplet states can produce delayed emission of lower energy (longer wavelength) phosphorescence with variable lifetimes. Since the triplet states can be efficiently quenched by oxygen, the changes in lifetime dynamics of the phosphorescence can be used to determine partial pressures of oxygen at local environment in real time. The developed probes have been efficiently delivered to the interior of cells by encapsulating at the core of polymeric micelles made of amphiphilic block copolymers.
2. ATP Probes
Adenosine-5′-triphosphate (ATP) is the common source of energy in the cell. The need for energy drives the rate of oxygen consumption and glycolysis in a cell, and the combination of oxidative phosphorylation and glycolysis determine overall ATP synthesis. Accurately measuring ATP levels, in conjunction with lactate production and oxygen consumption rate provide a comprehensive picture of the relative contributions of oxidative phosphorylation and glycolysis to overall energy production in genetically defined cell lines. We have developed novel live-cell intracellular ATP sensors selective over DNA, RNA, GTP, and ADP. Most fluorescent probes are quenched upon aggregation; however, the class of sensor used for this study brightens due to aggregation-induced emission (AIE) in the presence of ATP. Developed sensors have been used to test hypothesis that adaptation to hypoxia can drive adoption of glycolytic phenotype in cells along cancerous progression pathway. Cellular uptake and viability have been demonstrated by fluorescence imaging, and heterogeneity and distribution of ATP in live cells were measured.
Completed Projects
1. Development of emissive molecular oxygen, pH, and ATP sensors for single cells (MLSC-NIH).
Research Highlight
Multi-color and multi-photon imaging probes: A series of fluorescent AIE probes with different colors encapsulated in polymeric micelles have been developed. Compared to their virtually nonemissive properties in organic solutions, the fluorescence intensity of these AIE dyes in micelles increase significantly due to the spatial confinement that restricts intramolecular motions of dyes and their compatibility with the hydrophobic core of polymeric micelles. The effect of the chemical structure of the AIE dyes and micelle cores on the photophysical properties the dye-doped micelles have been systematically studied.
The highest fluorescence quantum yield of 62% has been achieved by encapsulating a green emitting dye HPS in the micelles. Intracellular modulation of color of emission has been achieved by FRET between a green emitting donor AIE dye and a red emitting acceptor AIE dye co-encapsulated in polymeric micelles. Efficient energy transfer (>99%) and high amplification of emission (as high as 8 times) from the red emitting NPAFN acceptor has been achieved by spatially confining the HPS/NPAFN FRET pair at the hydrophobic core of polymeric micelles. Successful intra cellular delivery of the micelles has been demonstrated by live cell imaging of RAW 264.7. Minimal cytotoxicity of these materials has been determined by cell viability assay (Figure). A series of new water-soluble red fluorescent two-photon absorbing (2PA) dyes have been developed also, and applied for high resolution spatial imaging with deep tissue penetration. The developed probes have been applied for two-photon induced fluorescence ion sensing, FRET imaging, and photodynamic therapy using polymeric micelles as intracellular delivery vehicles.
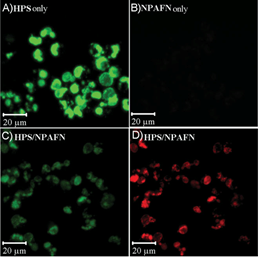
Confocal images of RAW cells incubated with A) HPS encapsulated PMAA-b-PS micelles using donor channel, B) NPAFN encapsulated PMAA-b-PS micelles using FRET channel, C,D) HPS/NPAFN-encapsulated PMAA-b-PS micelles using donor channel and FRET channel, respectively. The incubation time is 16 h. Journal cover illustration shown on right.
Related Publications
- “Utilization of micelles formed from poly(ethylene glycol)-block-poly(e-carpolactone) block copolymer as nanocarriers to enable hydrophobic red two-photon absorbing (2PA) emitters for cells imaging.” Yanqing Tian, Wen-Chung Wu, Ching-Yi Chen, Sei-Hum Jang, Meng Zhang, Tim Strovas, Judy Anderson, Brad Cookson, Yongzhong Li, Deirdre Meldrum, Wen-Chang Chen, Alex K.-Y. Jen. Journal of Biomedical Materials Research Part A 2010, 93A, 1068.
- “2,1,3-Benzothiadiazole (BTD)-moiety-containing red emitter conjugated amphiphilic poly(ethylene glycol)-block-poly(e-caprolactone) copolymers for bioimaging” Yanqing Tian, Wen-Chung Wu, Ching-Yi Chen, Tim Strovas, Yongzhong Li, Yuguang Jin, Fengyu Su, Deirdre Meldrum, Alex K.-Y. Jen Journal of Materials Chemistry 2010, 20, 1728.
- “Enhancement of aggregation-Induced emission in dye-encapsulating polymeric micellesfor bioimaging.” Wen-Chung Wu, Ching-Yi Chen, Yanqing Tian, Sei-Hum Jang, Yuning Hong, Yang Liu, Rongrong Hu, Ben Zhong Tang, Yi-Ting Lee, Chin-Ti Chen, Wen-Chang Chen, Alex K.-Y. Jen, Advanced Functional Materials 2010, 20, 1413.
- “Tracking bacterial infection into macrophages by a novel red-emission pH sensor” Yuguang Jin, Yanqing Tian, Weiwen Zhang, Sei-Hum Jang, Alex K.-Y. Jen, Deirdre R. Meldrum, Analytical and Bioanalytical Chemistry 2010, 398, 1375.
- “Dually fluorescent sensing of pH and dissolved oxygen using a membrane made from polymerizable sensing monomers” Y. Q. Tian, B. R. Shumway, A. C. Youngbull, Y. Li, A. K.-Y. Jen, R. H. Johnson, D. R. Meldrum, Sensors and Actuators, B. Chemical 2010, 147, 714.
- “Using micro-patterned sensors and cell self-assembly for measuring the oxygen consumption rate of single cells” James R. Etzkorn,Wen-Chung Wu, Zhiyuan Tian, Prince Kim, Sei-Hum Jang, Deirdre R. Meldrum, Alex K-Y Jen, Babak A. Parviz, J. Micromech. Microeng. 2010, 20, 095017.
- “2-(2’-Hydroxyphenyl)benzoxazole-containing two-photon absorbing chromophores as sensors for zinc and hydroxide ions” Y. Q. Tian, C.-Y. Chen, C.-C. Yang, A. C. Young, S.-H. Jang, W.-C. Chen, A. K.-Y. Jen, Chemistry of Materials 2008, 20, 1977.
- “Hydrophobic chromophores in aqueous micellar solution showing large two-photon absorption cross-sections” Y. Q. Tian, C.-Y. Chen, Y.-J. Cheng, A. C. Young, N. M. Tucker, A. K.-Y. Je, Advanced Functional Materials 2007, 17, 1691.
- “Two-photon absorbing block copolymer as a nanocarrier for porphyrin – energy transfer and singlet oxygen generation in micellar aqueous solution” C.-Y. Chen, Y. Q. Tian, Y.-J. Cheng, A. C. Young, J.-W. Ka, A. K.-Y. Jen, J. Am. Chem. Soc. 2007, 129 (23), 7220.
- “Cross-conjugated polymers with large two-photon absorption cross-sections for metal ion sensing” F. Huang, Y. Q. Tian, C.-Y. Chen, Y.-J. Cheng, A. C. Young, A. K.-Y. Jen, J. Phys. Chem. C, 2007, 111, 10673.
- “New 2-(2-Hydroxyphenyl)benzoxazole-containing poly(N-isopropylacrylamide) copolymer as pH, zinc ion and temperature sensor” C.-C. Yang, Y. Q. Tian, C.-Y. Chen, A. K.-Y. Jen, W.-C. Chen, Macromol. Rapid. Commun. 2007, 28, 894.
Current Team Members: Dr. Sei-Hum Jang and Jeffrey Yang
Related Interests and Keywords: fluorescence, phosphorescence, two-photon absorption, aggregation-induced emission, fluorescence resonance energy transfer, polymeric micelle, amphiphilic block copolymer, and plasmonics.