
cid regulated cell therapy for the treatment of cancer
CID-Regulated Red Cell Production. We have used CIDs, in combination with a derivative of the thrombopoietin receptor (F36VMpl), to direct the Epo-independent production of red blood cells in mice in dogs followed for longer than six years (reference 16 and data not shown) and in human CD34+ cells ex vivo and following transplantation into immune deficient mice. Representative results from one of our mouse studies are shown in Figure 2 (below). While our vector expresses F36VMpl in all hematopoietic lineages, the predominant effect of CID administration is to enhance red blood cell production.
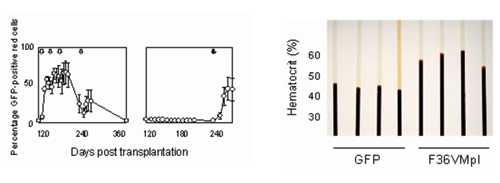
Figure 2: CID regulated erythropoiesis. Left panel: Effect of AP20187 administration on mean percentages of GFP+ red cells (RBCs), in 5 mice transplanted with congenic marrow cells transduced with an MSCV-based vector encoding both F36VMpl and a GFP marker and treated with 4 separate courses of AP20187 beginning 4 months post transplant (left) or monitored for 8 months, then treated with AP20187 (right). Arrows indicate 3 day courses of AP20187 (10 mg/kg/day). Error bars denote standard deviations. Right panel: Spun hematocrits from 8 mice one week following a 7 day course of AP20187. GFP: Mice transplanted with marrow cells transduced with a control vector, with hematocrits ranging from 40-46%; F36VMpl: Mice transplanted with the F36VMpl vector had hematocrits ranging from 56-62% (p=0.003).
CID-induced erythrocytosis can down-regulate endogenous Epo. One of the theoretical benefits of using CIDs in patients with cancer is the decline in endogenous Epo that is expected to accompany a CID-triggered increase in red cells. We have demonstrated that this prediction holds true in an anemic mouse model. Pyruvate kinase (pk)-deficient mice have a marked hemolytic anemia and dramatically reduced red cell survival. We established a chimeric mouse model in which 90% of marrow cells were of pk-deficient host origin, whereas the remaining 10% of cells originated from normal donor marrow cells that had been transduced with a vector encoding F36VMpl immediately prior to transplantation. CID administration specifically directed the expansion of the normal donor erythrocytes, promoting an increase in circulating red cells, (Figure 3A, below), and a decline in circulating Epo levels (Figure 3B, below).
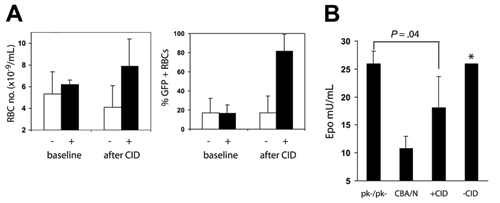
Figure 3. Epo levels decline following CID administration. Three of six pk deficient mice containing ~10% MSCVGFPiresF36VMpl transduced normal donor marrow cells were assigned to treatment with AP20187 10 µg/kg, 3 days a week for 2 weeks, then every day for 2 weeks, while the remaining 3 mice provided non-CID treated controls. (A) Erythrocyte numbers and the percentage of GFP-positive erythrocytes increased in CID treated mice (+) relative to untreated mice (-). (B) Epo levels were measured in the 6 mice, 3 CBA/N mice, and 3 CBA-Pk-1slc/Pk-1slc mice (PK-/PK-). Treated mice had Epo levels between those of CBA/N mice and those of CBA-Pk-1slc/Pk-1slc mice. *Data from 1 of the untreated mice with an Epo level of 570 mU/mL was not included in the bar chart.
CID-dependent red cell production is Epo-independent. To test the premise that CID- stimulated erythropoiesis is not dependent on the presence of Epo, F36VMpl-transduced human CD34+ cord blood (CB) cells were evaluateded for their ability to generate red cells in response to CID treatment, in the presence or absence of the Epo antagonist, soluble recombinant human EpoR (srhEpoR). Results are shown in Figure 4 (below).
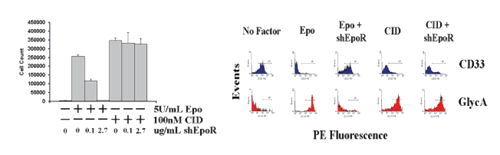
Figure 4: CID-dependent red cell production is not affected by Epo blockade. Cord blood CD34+ cells were transduced with a lentivirus vector encoding F36VMpl, then cultured in the presence (+) or absence (-) of Epo (5 U/ml), AP20187 (100 nM), and the competitive Epo antagonist, soluble recombinant human EpoR (at the concentration indicated). Cells counts (left panel) and flow cytometry (right panel were performed on day 12. Results show that AP20187 dependent red cell production is not impaired by Epo ablation.
Cells cultured in the absence of Epo did not proliferate (Figure 4, above) and retained expression of the myeloid marker CD33 (Right Panel, above). Epo (5 U/mL) induced proliferation (66.2-fold in 12 days) and CD33 expression was lost as the cells differentiated into glycophorin A+ erythroid cells. Addition of a competitive inhibitor of Epo binding (soluble human EpoR extracellular domain [shEpoR]) at a concentration of 2.7 μ/mL completely blocked Epo-dependent proliferation and, similar to cells cultured without Epo, CD33 expression persisted. Addition of CID (100nM AP20187) (without Epo) promoted cell expansion (89.8-fold in 12 days) and differentiation as glycophorin A+ erythroid cells, findings that were unchanged in the presence of shEpoR. These data suggest that F36VMpl does not require Epo signaling to support the proliferation and differentiation of human erythroid progenitor cells.
Advantages of CID regulated red cell production in patients with cancer. Developing methods for converting red cell production from dependency on Epo to dependency on CIDs would provide at least 3 advantages in patients with cancer by i) circumventing the need for exogenous Epo; ii) reducing endogenous levels of Epo via feedback inhibition of renal Epo secretion (Figure 5, below); and iii) opening the potential for combining with Epo antagonists to achieve total ablation of Epo signaling, analogous to androgen blockade for prostate cancer or estrogen blockade for breast cancer, possibly providing a new way to treat a wide range of malignancies. Consistent with the strategy, studies in mice indicate that Epo blockade may provide a new way of treating cancer. A schematic depiction of the goal for our proposed approach is shown in Figure 5, below.
Erythropoietin Blockade for the Treatment of Cancer
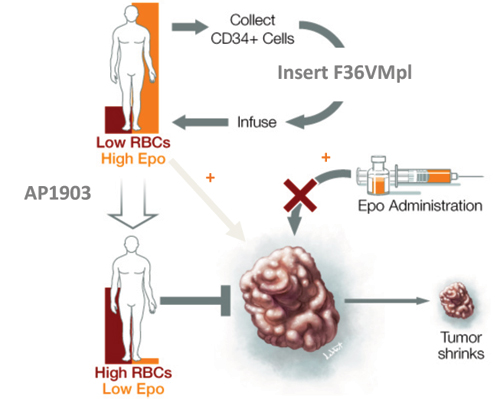
Figure 5. Epo blockade for the treatment of cancer. Cancer may be sustained not only by exogenous Epo, but also by endogenous levels of Epo that accompany anemia. F36VMpl gene transfer followed by AP1903 administration provides a mechanism for the Epo independent regulation of red cell production, avoiding exogenous Epo administration, and reducing endogensous Epo levels. This approach can also be combined with Epo/EpoR antagonists, allowing for the complete ablation of Epo signaling (not shown).
Figure 6. Potential applications of pharmacologically regulated cell therapy.