Mixed Conduction in Polymers
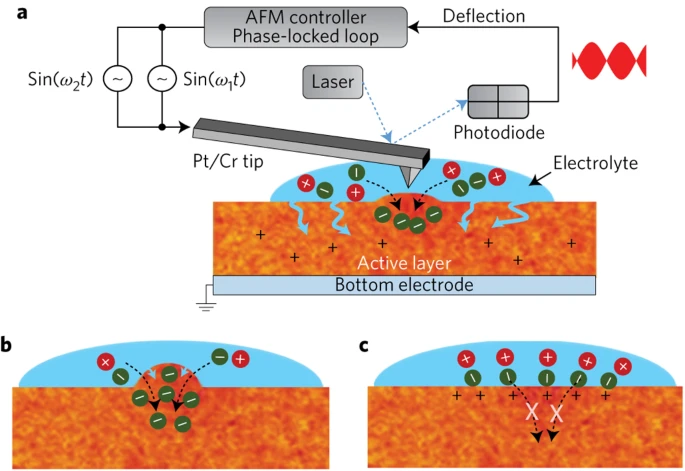
Recently, there has been a surge of interest in the field of organic mixed ionic-electronic conductors (OMIECs). These materials can transport both ions and charge carriers, and OMIECs offer significant advantages for biosensor or supercapacitors. In transistors they act as effective transducers that can convert ions from an aqueous electrolyte (e.g. a salt solution like KCl) into electrical current by doping (or dedoping) the channel. A key advantage of OMIECs over inorganic materials is that they absorb ions throughout the volume. In precise terms, OMIECs exhibit very high “volumetric capacitance,” which enables sensitive detection of small currents at voltages amenable for use in the body (<1 V). Silicon sensors, on the other hand, require much high voltages or much higher signal.
In our lab, we study a wide range of problems in OMIECs. These projects straddle physical chemistry, polymer physics, device physics, optical microscopy, and imaging. For example, our earliest foray into this field involved studying how the swelling in a polymer is inversely correlated with its crystallinity by using atomic force microscopy. We expanded this work to study new types of polymers in collaboration with Christine Luscombe’s lab, as well as other polymers from different labs like Sam Jenekhe’s group. A key part of our focus is on kinetics — how quickly ions move through a film. We are studying this via spectroelectrochemistry, optical microscopy, X-ray diffraction/scattering, and scanning probe microscopy. Related to this work is a basic analysis of new polymers (both n-type and p-type), new devices, and new physical chemistry.
For more information, here are a few key papers from our lab in this area:
Giridharagopal, R.; Flagg, L. Q.; Harrison, J. S.; Ziffer, M. E.; Onorato, J.; Luscombe, C. K.; Ginger, D. S. Electrochemical Strain Microscopy Probes Morphology-Induced Variations in Ion Uptake and Performance in Organic Electrochemical Transistors. Nat. Mater. 2017, 16 (7), 737–742. https://doi.org/10.1038/nmat4918.
Flagg, L. Q.; Bischak, C. G.; Onorato, J. W.; Rashid, R. B.; Luscombe, C. K.; Ginger, D. S. Polymer Crystallinity Controls Water Uptake in Glycol Side-Chain Polymer Organic Electrochemical Transistors. J. Am. Chem. Soc. 2019, 141 (10), 4345–4354. https://doi.org/10.1021/jacs.8b12640.
Bischak, C. G.; Flagg, L. Q.; Yan, K.; Rehman, T.; Davies, D. W.; Quezada, R. J.; Onorato, J. W.; Luscombe, C. K.; Diao, Y.; Li, C.-Z.; Ginger, D. S. A Reversible Structural Phase Transition by Electrochemically-Driven Ion Injection into a Conjugated Polymer. J. Am. Chem. Soc. 2020, 142 (16), 7434–7442. https://doi.org/10.1021/jacs.9b12769.
