In addition to the famous impacts of atmospheric carbon on global warming, there is an equally worrisome effect of carbon dioxide on the oceans: ocean acidification (or OA for short). Here at I2SEA we have a series of resources about OA, including Our Acidifying Ocean, which is an introductory activity and virtual lab (note: now mobile compatible!).
A student suggested that we start a conversation topic about OA, and here it is! Have you heard about Ocean Acidification? If so, are you worried about it? How do you think we can best raise awareness about it among your fellow students and the general public? Have you seen particularly good links or films about OA that you would like to share? What are you doing to address the problem?
Ocean Acidification >
Combat Ocean Acidification! Save the Creatures!

Combating ocean acidification is crucial for the health of marine ecosystems and the livelihoods that depend on them. As carbon dioxide levels rise, more of this greenhouse gas is absorbed by the oceans, leading to a decrease in pH levels and disrupting the delicate balance of marine life. Coral reefs, shellfish, and various marine organisms struggle to build their calcium carbonate structures in increasingly acidic waters, threatening biodiversity and fisheries. Effective strategies to address ocean acidification include reducing carbon emissions, promoting sustainable practices, and restoring coastal habitats like mangroves and seagrasses, which can help buffer changes in pH. Collaborative global efforts, research, and public awareness are vital to mitigate the impacts of ocean acidification, ensuring the resilience of our oceans for future generations.

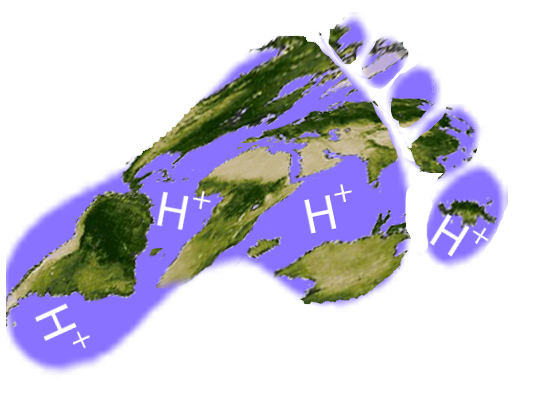
Ocean acidification is expected to have negative overall effects on many marine species. This could alter marine food chains and food supply to humans. Ocean acidification occurs when carbon dioxide (CO2) is absorbed rapidly into the ocean. It reacts with water molecules (H2O) to form carbonic acid (H2CO3). This compound then breaks down into a hydrogen ion (H+) and bicarbonate (HCO3-). These hydrogen ions decrease seawater pH.