

| Home | |
| Research | |
| Publications | |
| AAV Serotype Reagents | |
| Foamy Reagents | |
| Lab Members | |
| Positions | |
| Related Links | |
| Contact Us | |
| For Lab Members | |
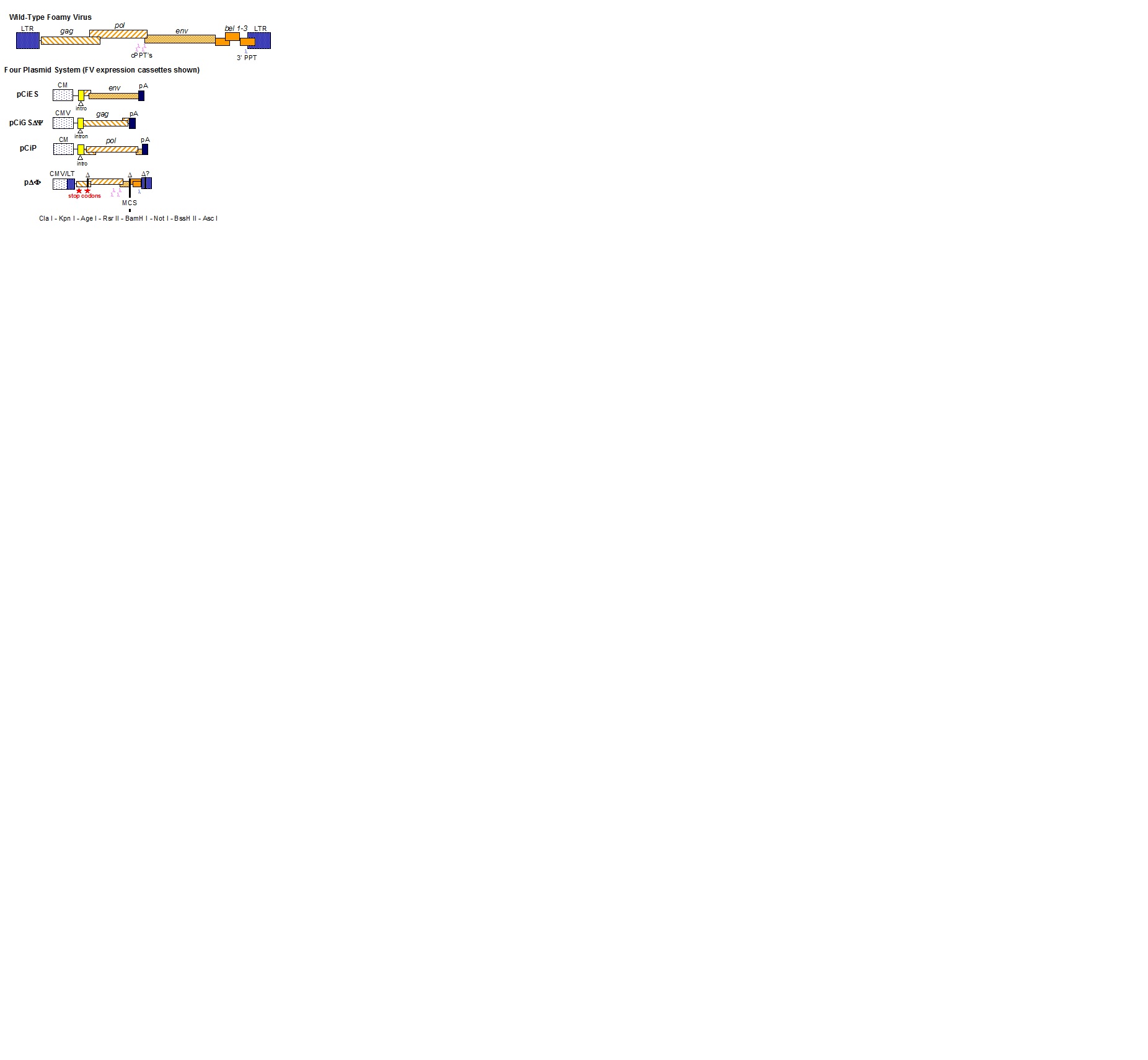
ΔΦ Vectors
ΔΦ vectors are "Deleted Foamy" virus vectors that only retain essential cis-acting viral sequences. The 4 plasmids required to make ΔΦ vectors are pCiES, pCiGSΔΨ, pCiPS and pΔΦ. The first three plasmids are helper constructs expressing Env, Gag, and Pol respectively. pΔΦ is a deleted foamy vector backbone with a polylinker for you to insert a transgene cassette. A description of the plasmids has been published (Trobridge et al., 2002). Note however, that pCiGSΔΨ is a newer version of the pCiGS Gag expression plasmid described in that paper, with a more complete deletion in the packaging signal (Josephson et al., 2004). All the plasmids encode ampicillin resistance. See separate links for an FV Vector Production Protocol and the sequences of plasmids pCiES, pCiGSΔΨ, pCiPS and pΔΦ (accurate as far as we know). Because FV vectors have cDNA genome, they can be titered by a qPCR protocol to determine the genome-containing particle number. This qPCR protocol requires a control plasmid called pACT5FVMF(f) (Hendrie et al., 2008).
NIFV Vectors
Non-Integrating Foamy Virus (NIFV) vectors are similar to integrating ΔΦ vectors, except they are produced with a helper plasmid that contains mutations in the DDE catalyic core motif of the integrase sequence, and therefore remain as untintegrated episomes in transduced cells (Deyle et al., 2010). In this packaging system, plasmid pCiPS-NIFV contains 2 integrase mutations and replaces pCiPS as the pol-expressing helper plasmid. We also recommend using a vector plasmid that includes these same two mutations in the cis-acting region, in order to prevent a recombination event from correcting the integrase mutations. An example is pNIFV, which is similar to pΔΦ, but contains these two integrase mutations. See separate links for the sequences of plasmids pNIFV-CiPS and pNIFV.
References
| Bauer, T.R., Jr., Allen, J.M., Hai, M., Tuschong, L.M., Khan, I.F., Olson, E.M., Adler, R.L., Burkholder, T.H., Gu, Y.C., Russell, D.W., et al. (2008). Successful treatment of canine leukocyte adhesion deficiency by foamy virus vectors. Nat Med 14, 93-97. | |
| Deyle, D.R., Khan, I.F., Ren, G., and Russell, D.W. (2013). Lack of genotoxicity due to foamy virus vector integration in human iPSCs. Gene Ther 20, 868-873. | |
| Deyle, D.R., Li, Y., Olson, E.M., and Russell, D.W. (2010). Nonintegrating foamy virus vectors. J Virol 84, 9341-9349. | |
| Gharwan, H., Hirata, R.K., Wang, P., Richard, R.E., Wang, L., Olson, E., Allen, J., Ware, C.B., and Russell, D.W. (2007). Transduction of human embryonic stem cells by foamy virus vectors. Mol Ther 15, 1827-1833. | |
| Hendrie, P.C., Huo, Y., Stolitenko, R.B., and Russell, D.W. (2008). A rapid and quantitative assay for measuring neighboring gene activation by vector proviruses. Mol Ther 16, 534-540. | |
| Josephson , N.C. , Trobridge, G., and Russell, D.W. (2004). Transduction of long-term and mobilized peripheral blood derived NOD/SCID repopulating cells by foamy virus vectors. Hum. Gene Ther. 15:87-92. | |
| Josephson , N.C. , Vassilopoulos, G., Trobridge, G.D., Priestley, G.V., Wood, B.L., Papayannopoulou, T., and Russell, D.W. (2002). Transduction of human NOD/SCID-repopulating cells with both lymphoid and myeloid potential by foamy virus vectors. Proc. Natl. Acad. Sci. USA 99:8295-8300. | |
| Kiem, H.P., Allen, J., Trobridge, G., Olson, E., Keyser, K., Peterson, L., and Russell, D.W. (2007). Foamy virus-mediated gene transfer to canine repopulating cells. Blood 109, 65-70. | |
| Ohmine, K., Li, Y., Bauer, T.R., Jr., Hickstein, D.D., and Russell, D.W. (2011). Tracking of specific integrant clones in dogs treated with foamy virus vectors. Hum Gene Ther 22, 217-224. | |
| Russell, D.W., and Miller, A.D. (1996). Foamy virus vectors. J. Virol. 70: 217-222. | |
| Trobridge, G., Josephson, N., Vassilopoulos, G., Mac, J, and Russell, D.W. (2002). Improved foamy virus vectors with minimal cis-acting sequences. Mol. Ther. 6:321-328. | |
| Trobridge, G.D., Miller, D.G., Jacobs, M.A., Allen, J.A., Kiem, H.P., Kaul, R., and Russell, D.W. (2006). Foamy virus vector integration sites in normal human cells. Proc. Natl. Acad. Sci. USA 103:1498-1503. | |
| Vassilopoulos, G., Trobridge, G., Josephson , N.C. , and Russell, D.W. (2001). Gene transfer into murine hematopoietic stem cells with helper-free foamy virus vectors. Blood 98:604-609. |