Modulation of Metamorphosis by Biogenic Amines
Tony Prires, Ph.D., Dickinson College
For many decades FHL has been a center of research on the beautiful and diverse larvae of marine invertebrates. Most of the 30+ phyla, or major taxonomic categories of animals, have larval forms that metamorphose into a dissimilar adult. In the marine environment, this metamorphosis is often triggered by a sensory signal that is related to adult habitat. As a neurobiologist, I want to understand how neurosensory mechanisms interact with developmental programs – particularly because such mechanisms are likely to be ancient and operative in some way in most animal life.
Much of my work has been about the modulation of metamorphosis by biogenic amines. These signaling molecules, released by nerve cells, influence behavior in all animals (including humans). Often they act to adjust the rate or pattern of activity of a neural circuit, or change the sensitivity of a sensory system to external stimuli. I have found that one of these neuromodulators, dopamine, makes larvae of marine snails more sensitive to natural environmental chemical cues that induce metamorphosis. However, all of these experiments have been done in highly unnatural laboratory conditions in which other sensory influences have been taken away. We know very little about how the nervous system regulates metamorphosis in nature.

To really understand metamorphosis, it will be necessary to study it in the context of multiple interacting internal and external cues. For example, my students and I have recently shown that brief mechanical stimulation makes snail larvae metamorphose more readily in response to a chemical cue that comes from conspecific adults. We have also identified candidate mechanosensory (touch-sensitive) neurons that contain a marker for dopamine, the neuromodulator that we have implicated in the control of metamorphosis. An attractive hypothesis is that these mechanosensory neurons are the source of dopamine that is released when larvae interact with their tactile environment. However, neurobehavioral study of metamorphosis is difficult because it occurs while larvae are crawling. It is hard to record electrical activity from the nervous system and measure behavior in small moving animals that will not stay in a video frame. Lack of stable imaging also precludes the use of fluorescent probes that mark internal physiological events that occur during metamorphosis.
To be able to image crawling, metamorphosing 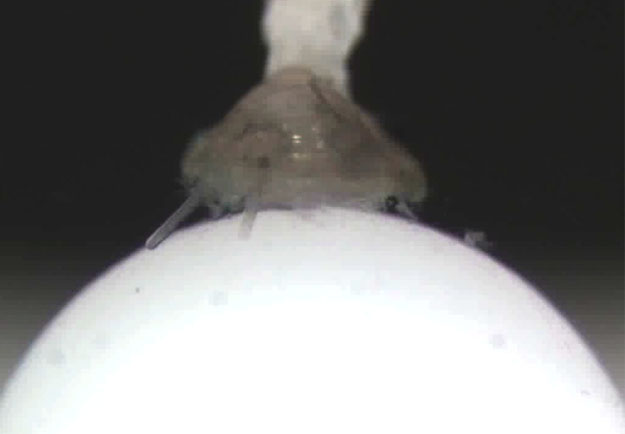 snail larvae, I built a prototype “larval treadmill” at FHL in the summer of 2011. I was fortunate to have access to Adam Summers’ new computer numerical control (CNC) milling machine for precise fabrication of the tiny cup that supports the rotating ball upon which the larva crawls (photo). The larva in the photo is a veliger of the gastropod, Crepidula fornicata, about 1.2 mm body length – big for a larva but small for an animal to be studied in this way. In addition to the CNC milling machine, the stockroom’s stash of old junked microscope parts also proved to be a most valuable resource. In the near future I hope to build the treadmill into a flow chamber and add fluorescence imaging capability, so that we can ask and answer a host of new questions about neurobiology and behavior of marine larvae.
snail larvae, I built a prototype “larval treadmill” at FHL in the summer of 2011. I was fortunate to have access to Adam Summers’ new computer numerical control (CNC) milling machine for precise fabrication of the tiny cup that supports the rotating ball upon which the larva crawls (photo). The larva in the photo is a veliger of the gastropod, Crepidula fornicata, about 1.2 mm body length – big for a larva but small for an animal to be studied in this way. In addition to the CNC milling machine, the stockroom’s stash of old junked microscope parts also proved to be a most valuable resource. In the near future I hope to build the treadmill into a flow chamber and add fluorescence imaging capability, so that we can ask and answer a host of new questions about neurobiology and behavior of marine larvae.

