HIV and Co-Infections Through the Lifecycle Scientific Priority Area
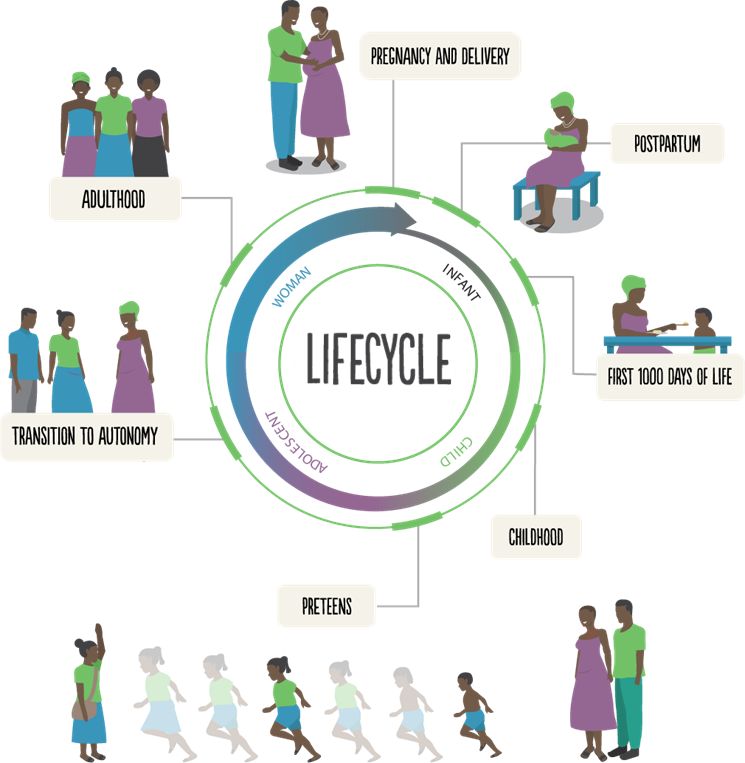 The Challenge
The Challenge
Women, adolescents, and children bear a disproportionate burden of HIV prevalence, incidence, and associated mortality globally. HIV and co-infections — including tuberculosis (TB) and viral infections — present unique health risks to children, adolescents, and women and have interconnected roots of susceptibility and interaction in an individual’s body. Without interventions, the implications of living with HIV and co-infections can persist throughout the lifecycle and into the next generation.
Our Response
We have cultivated expertise in HIV, TB, and other viral infections, particularly as they relate to women, adolescents, and children in resource-limited settings. We leverage that expertise to commit to a vision of transforming testing, diagnostic, and treatment approaches through integrated, innovative, and impactful research that consciously adopts the perspectives of these diverse age groups. Our research touches all areas of the translational scientific spectrum from making basic and clinical scientific discoveries to applying implementation science to deliver proven interventions that improve health outcomes. We aim to serve as a hub for information, expertise, and collaboration in infectious disease research across the lifecycle.
Areas of Focus
Women
We are committed to preventing transmission of HIV, TB, and other viral infections from mother-to-child by prioritizing biologic determinants of conveyance, mobile health (mHealth) interventions to maximize prevention intervention adherence, and systematic evaluations of the programs currently in place. We further prioritize preventing and detecting HIV acquisition for pregnant and postpartum women. We center our research efforts for this population on pre-exposure prophylaxis (or PrEP) in pregnancy and repeat HIV testing.
Adolescents
Meeting the unique needs of adolescents living with HIV, TB, and other pathogens necessitate improvements to the quality, level of engagement, and adherence to HIV care. We make these improvements by conducting research using simulated patient training, qualitative studies, continuing quality improvement, program evaluations, mHealth, social media, schools, and service delivery evaluations.
Children
Interventions during childhood have a substantial impact on the quality of HIV care and management of other illnesses throughout the remainder of a child’s life. That’s why our research in this population area is focused on expediting diagnosis, understanding pathogenesis, optimizing treatment and long-term outcomes, and reducing mortality. These research efforts focus on HIV testing models, financial incentives, immunologic and virologic determinants of acquisition and disease, co-infections, HIV-exposed uninfected children’s adherence and disclosure within care, treatment interruption, and accelerated ART.

Collaborators
Faculty Directors

MD, PhD
Professor
Global Health, Epidemiology, Medicine, and Pediatrics

MD, MPH
Assistant Professor
Global Health and Medicine

MBChB, MPH, MS, PhD
Research Scientist
Kenyatta National Hospital
Affiliate Assistant Professor
Global Health

MS, PhD
Associate Professor
Global Health
Adjunct Associate Professor
Epidemiology

MPH, PhD
Assistant Professor
Global Health
Faculty

MPH, PhD
Senior Research Scientist
Global Health

MS, PhD
Senior Research Scientist
Global Health

MS, PhD
Clinical Assistant Professor
Global Health;
Senior Research Scientist
Kenyatta National Hospital

MPH, PhD
Assistant Professor
Global Health and Epidemiology [Adjunct]

MPH, PhD
Assistant Professor
Global Health and Epidemiology


MBChB, MMed, MPH
Affiliate Associate Professor
Global Health
Head of Research and Programs
Kenyatta National Hospital

MS, PhD
Clinical Associate Professor
Global Health

Mbori-Ngacha
MBChB, MMed, MPH
Senior HIV Specialist
UNICEF

PhD
Assistant Professor
Global Health


MBChB, MPH
Professor and Former Chair
University of Nairobi Paediatrics and Child Health

MPH, PhD
Assistant Professor
Nursing

PhD
Assistant Professor
Global Health

MBChB, MMed, MPH
Associate Professor
University of Nairobi Paediatrics and Child Health
Technical Advisors


MPH, PhD
Executive Director
Impact Research and Development Organization

MPH, PhD
Research Professor
Global Health and Obstetrics & Gynecology

MD, PhD
Professor
Global Health, Epidemiology, and Medicine

MPH, PhD
Professor
Global Health and Child, Family, and Population Health Nursing



MBChB, MMed, MPH
Professor
University of Nairobi Paediatrics and Child Health

MD, PhD
Assistant Professor
Global Health Implementation Science

Research Professor
Biostatistics and Global Health [Adjunct]
Staff

MS
Research Coordinator

MPH
Research Scientist

MPH
Research Coordinator

MPH, PhDc
Research Coordinator

MPH, PhD
Data Manager/Analyst

Kenya Finance Manager

MPA
Grant and Program Manager

BSW
Research Coordinator

BA
Research Coordinator

MPH
Senior Data Manager
Trainees

MPH
PhD Candidate
Epidemiology

MPH, PhD
Postdoctoral Scholar

MPH
PhD Student
Global Health

MPH
PhD Candidate
Predoctoral Fellow
Global Health


MBChB, MPH
PhD Student
Jomo Kenyatta University

MD
Infectious Disease Fellow

MPH
PhD Student
Epidemiology

MPH, PhD
Postdoctoral Scholar

MPH Student
Epidemiology

MBChB, MMed
MPH Student
Global Health

MBChB, MPH, PhD
Research Scientist
Kenyatta National Hospital

MBChB
MPH Student
Global Health


MPH, PhD
Postdoctoral Scholar

MBChB, MMed
Tutorial Fellow and Pediatrician

MBBS, MS
Medical Epidemiologist
Kisumu County Department of Health

PhD
Postdoctoral Scholar

Our Research
Improving HIV Testing and Care
Transitioning from Pediatric to Adult HIV Care in Kenya (ATTACH)
ATTACH aims to evaluate rates and co-factors of effective transition in HIV treatment programs in Kenya and to adapt, implement, and evaluate an Adolescent Transition Package (ATP) that combines an adapted US-based transition tool and a disclosure tool associated with improved disclosure outcomes in Namibia.
Nested in this study, Danae Black is leading a NIH-funded Administrative Diversity Supplement study titled, “Evaluating TB prevention strategies for adolescents and young adults in Kenya,” to identify individual and facility-level determinants of ICF, TPT initiation and completion.
Sponsor: National Institute of Child Health and Human Development (NICHD) (5R01HD089850)
Award Years: 08/01/2016 – 06/30/2021
Principal Investigator: Grace John-Stewart
Co-Investigators: Jennifer Slyker, Dalton Wamalwa, Pamela Kohler, Gabrielle O’Malley, Kristin Beima-Sofie
Trainees: Danae Black
Click here to view the study protocol.
Click here to view the study charter agreement.
Click here to view downloaded study materials. (Please email Dr. Kristin Beima-Sofie at beimak@uw.edu for the password to access this page)
Planning the mPACT Trial: mHealth Strategies for the Pediatric to Adult HIV Care Transition
This planning grant will pilot a mHealth framework that uses a combination of group peer support and one-to-one communication with a healthcare provider specifically trained in youth HIV care.
Sponsor: National Institutes of Health (3R34MH114834)
Award Years: 07/01/2018 – 04/30/2021
Principal Investigator: Brandon Guthrie
Co-Investigators: Keshet Ronen, Jennifer Unger, Megan Moreno
Data-informed Stepped Care to Improve Adolescent HIV Outcomes (DiSC)
Adolescents (ages 10-19) experience disproportionately low retention, adherence, and viral suppression compared to other age groups. The DiSC study aims to implement a combination of data-driven interventions using a stepped care model to improve adolescent HIV engagement and clinical outcomes in Kenya. This generalizable systematic approach to deliver differentiated adolescent HIV care that integrates with diverse HIV care programs contributes to the acceleration of progress towards achieving 90-90-90 targets for adolescents.
Sponsor: National Institutes of Health (UG3HD096906)
Award Years: 09/01/2018 – 08/31/2020
Principal Investigators: Grace John-Stewart, Pamela Kohler
Co-Investigators: Barbra Richardson, Kristin Beima-Sofie, Katherine Wilson
Staff: Jessica Dyer
Trainees: Jill Neary
Developing a Data-Informed Caregiver Intervention to Improve Adolescent HIV
Reaching 95-95-95 targets for adolescents living with HIV (ALHIV) will require the development and diffusion of evidence-based interventions that address barriers to treatment and care. Primary caregivers of adolescents are an untapped support resource. Comprehensive, data-driven interventions that are responsive to caregiver, adolescent, healthcare worker, and policy-maker needs could substantially improve adolescent HIV outcomes. The proposed study will leverage an ongoing UG3/UH3 grant focused on identifying risk factors for viral non-suppression and loss-to-follow-up and optimizing health service provision for adolescents to develop an intervention that will empower caregivers to support ALHIV in Kenya.
Sponsor: UW/Fred Hutch Center for AIDS Research (CFAR) New Investigator Award
Award Years: 07/01/2019 – 6/30/2020
Principal Investigator: Kristin Beima-Sofie
Co-Investigators: Keshet Ronen, Irene Njuguna
Mentors: Grace John-Stewart, Jane Simoni, Gabrielle O’Malley, Dalton Wamalwa
WhatsApp Focus Group and Respondent-Driven Sampling: Novel Approaches to Engage Diverse Adolescents
This one year project aims to test new technology-based approaches to engage adolescents who do not typically seek HIV preventative and treatment services, and determine whether adolescents recruited via WhatsApp are demographically distinct from those identified in a clinical setting. This project will test respondent-driven sampling—a social network-based strategy used to collect data from hard-to-reach populations who may fear being identified with stigmatized behavior, such as injection drug users, female sex workers, and men having sex with men—in hopes of reaching adolescents from diverse backgrounds and health-seeking behaviors. They will also compare the WhatsApp virtual focus groups to traditional in-person focus groups in terms of content and depth of responses, and costs.
Sponsor: UW/Fred Hutch Center for AIDS Research (CFAR) International Pilot Award
Award Years: 01/2018 – 01/2019
Principal Investigator: Anjuli Wagner
Co-Investigator: Irene Njuguna
Financial Incentives to Increase Update of Pediatric HIV Testing (FIT)
This pilot study will evaluate the acceptability, feasibility, and costs of offering financial incentives to HIV-infected parents to motivate testing of their HIV-exposed children. 60 parents of children <12 years old who are utilizing HIV treatment services at Kenyatta National Hospital (Nairobi, Kenya) will be randomized to receive $5, $10, or $15 cash payment contingent upon completing pediatric HIV testing. A post-test questionnaire will evaluate acceptability, feasibility, and motivational mechanisms of the intervention. Data will be used to support the future submission of a larger R01-scale grant evaluating the effectiveness of the intervention.
Sponsors: UW/Fred Hutch Center for AIDS Research (CFAR) International Pilot Award and International AIDS Society Collaborative Initiative for Paediatric HIV Education and Research (IAS CIPHER)
Award Years: 01/16/2015 – 05/31/2017
Principal Investigator: Jennifer Slyker (CFAR), Irene Njuguna (IAS CIPHER)
Co-Investigators: Grace John-Stewart, Anjuli Wagner
Click here to view the study protocol.
Diagnostic Performance and Acceptability of Saliva-based HIV Testing in Children (STEP-UP)
This study aims to validate saliva-based HIV testing in children and to develop and test a video-based pre-test information session for outpatient HIV testing.
Sponsors: Thrasher Research Fund and UW/Fred Hutch Center for AIDS Research (CFAR) New Investigator Award
Award Years: 03/01/2017 – 02/28/2019
Principal Investigator: Grace John-Stewart (Thrasher), Anjuli Wagner (CFAR NIA)
Co-Investigators: Anjuli Wagner, Irene Njuguna
Technical Support on WHO HIV Testing Guidelines
We aim to support the World Health Organization (WHO) HIV Department Key Population and Innovative Prevention (KPP) unit’s work on HIV testing services. These tasks focus on three key areas: (1) supporting implementation of partner notification services (PNS) globally, (2) gathering and synthesizing evidence and conducting scoping reviews in order to prepare for the 2019 update to the WHO Consolidated Guidelines on HIV Testing Services, (3) inform implementation of HIV and syphilis testing strategies in maternal and child health programmes, and (4) guide programming for integration of HIV testing into family planning service delivery.
Sponsor: World Health Organization (PO202170778)
Award Years: 04/01/2018 – 12/31/2019
Principal Investigator: Alison Drake
Co-Investigators: Brandon Guthrie, Christine Khosropour, David Katz, Anjuli Wagner
Assessing Mother and Infant Antiretroval Exposure Using Hair Measures (Mother/Infant ART Expo)
The proposed study aims to quantify maternal-to-infant transfer during pregnancy for a variety of ARVs by employing a novel pharmacokinetic evaluation using maternal and neonatal hair in an unprecedented cohort. We will use validated assays developed by our group to measure ARV levels in hair samples collected at birth from mother-infant pairs enrolled in the Surveillance Monitoring for ART Toxicities Study in HIV-uninfected Children Born to HIV-infected Women (SMARTT Study), a Pediatric HIV/AIDS Cohort Study (PHACS)-funded prospective study designed to evaluate the effects of ARV exposure on infants born to HIV-infected mothers in the U.S. Our results will improve understanding of maternal-to-infant transfer for common ARVs used during pregnancy with ultimate implications for worldwide efforts to prevent perinatal HIV transmission with maximum efficacy and minimal toxicity.
Sponsor: National Institutes of Health (2R21AI138618)
Award Years: 06/06/2018 – 05/31/2020
Principal Investigator: Jillian Pintye
Infant Immune Mechanisms of HIV Reservoir Size and Decay (HIV PERSISTENCE)
The study will model longitudinal reservoir dynamics, and determine the effects of infant timing of HIV acquisition, ART timing, and the influence of infant immune activation, ADCC, and NK population characteristics on reservoir decline and size. These studies will provide novel data on reservoir dynamics during infancy and early childhood from sub-Saharan Africa and will elucidate potential influence of immune activation, ADCC, and natural killer phenotype in reservoir containment, and ultimately inform intervention strategies for improved long-term management and reservoir control in HIV infected children.
Sponsor: National Institutes of Health (5R01HD094718)
Award Years: 09/01/2017 – 06/30/2022
Principal Investigators: Grace John-Stewart, Dara Lehman
Co-Investigators: Sarah Benki-Nugent, Jennifer Slyker
Integration of stepped care for Perinatal Mood and Anxiety Disorders among Women Living with HIV in Kenya
The proposed studies offer an unprecedented opportunity to understand how HIV, co-infections, malnutrition and inflammation alter early life and long-term immune reactivity in African children. Results from this study have great potential to optimize vaccination strategies for children in areas of HIV prevalence, CMV and malnutrition.
Sponsor: National Institutes of Mental Health (R01MH133266)
Award Years: 09/01/2017 – 06/30/2022
Principal Investigators: John Kinuthia (Kenyatta National Hospital), Amritha Bhat, Keshet Ronen
Co-Investigators: Carol Levin, Barbra Richardson, Bryan Weiner, Anjuli Wagner
Preventing Mother-to-Child Transmission (PMTCT) for Women Living with HIV
Preventing, Detecting, and Treating Incident Maternal HIV Infection for PMTCT
This award supports leveraging data and archived data collected as part of Dr. Drake’s ongoing K01 study in Kenya, which aims to determine the optimal time to conduct repeat maternal HIV testing. The specific aims are to: 1) externally validate two HIV risk score tools among a cohort of 4600 pregnant/postpartum women, 2) measure maternal HIV viral load and prevalence of TDR among 100 women with incident HIV infection during pregnancy/postpartum, and 3) identify biological and behavioral correlates of maternal HIV infection during pregnancy and postpartum. Together, data from the K01 and R03 studies will provide preliminary data to prepare for a large, implementation science trial of repeat maternal HIV testing coupled with targeted screening to determine eligibility for PrEP.
Sponsor: National Institute of Health (2R03AI140922)
Award Years: 07/06/2018 – 06/30/2020
Principal Investigator: Alison Drake
Optimizing Repeat HIV Testing During Pregnancy and Postpartum for PMTCT (OPT)
This career development award focuses on HIV viral load and resistance testing among pregnant/postpartum women with incident maternal HIV infections.
Sponsor: National Institute of Allergy and Infectious Diseases (5K01AI116298)
Award Years: 07/10/2015 – 06/30/2020
Principal Investigator: Alison Drake
Mentors: Grace John-Stewart, Kenneth Sherr
Supporting the Implementation and Expansion of High Quality, Sustainable and Comprehensive HIV Prevention, Care and Treatment Programs in Faith-Based Organization Facilities in the Republic of Kenya under the Presidents Emergency Plan for AIDS Relief
This is a Collaborative Agreement to promote international research efforts and improve the health of Kenyans through medical and preventative health measures. The scope of work includes completion of study activities for the three program evaluations listed below. Specifically, this includes completion of data collection activities, data cleaning and analyses, dissemination of results through report writing, and manuscript development. The funding supports the following studies:
1. Impact of ART Adherence and Early Infant HIV Diagnosis on the Effectiveness of Option B+ in Kenya (Option B+)
2. Prevalence, Cofactors, and Types of Family Planning Methods Used by HIV-infected Women in HIV Care Programs in Kenya (FP)
3. Assessment of Adolescents HIV Care in Large HIV Treatment Programs in Kenya (PHASE)
Sponsor: Christian Health Association of Kenya (NU2GGH002034)
Award Years: 10/01/2018 – 03/31/2019
Principal Investigators: Grace John-Stewart, John Kinuthia
Co-Investigators: Sarah Benki-Nugent, Kristin Beima-Sofie, Alison Drake, Christine McGrath
Mobile WACH X: Evaluation of mHealth Strategies to Optimize Adherence and Efficacy of PMTCT/ART
This randomized trial was designed to determine the effect of a systematic provision of tailored one-way SMS or two-way SMS dialogue to control virologic suppression in HIV-infected peripartum women in Kenya.
Sponsor: National Institute for Health Child Health & Human Development (5R01HD090460)
Award Years: 05/01/2014 – 04/30/2021
Principal Investigators: Grace John-Stewart, John Kinuthia
Co-Investigators: Keshet Ronen, Jennifer Unger
Integrating a transdiagnostic psychological intervention in the care for adolescents and youth with HIV in Kenya (PROACT)
The investigators at the University of Washington will contribute expertise on adolescents living with HIV in African contexts and implementation science theoretical frameworks that guide study activities, and will assist in the application of implementation science tools and analysis of implementation science outcome data.
Sponsor: National Institute for Mental Health (R01MH133261)
Award Years: 05/01/2014 – 04/30/2021
Principal Investigators: Pamela Collins
Co-Investigators: Ferdinand Mukumbang, Anjuli Wagner, Shannon Dorsey, Brian Flaherty
Optimizing PrEP Delivery
PrEP Implementation for Mothers in Antenatal Care (PrIMA)
PrEP Implementation for Mothers in Antenatal Care (PrIMA) is a cluster-randomized trial to determine the best model for optimized PrEP delivery in pregnancy within maternal-child health systems.
Sponsor: National Institute of Health (5R01AI125498)
Award Years: 05/01/2016 – 04/30/2021
Principal Investigator: Grace John-Stewart
Co-Investigators: Jared Baeten, Ruanne Barnabas, Barbra Richardson
Staff: Julia Dettinger, Laurén Gomez
Click here to view the study protocol.
Evaluating Infant PrEP Exposure During Pregnancy and Breastfeeding (PRIMA-EXT)
The proposed study aims to quantify infant pre- and post-natal PrEP exposure and evaluate birth, bone, growth, and neurocognitive outcomes following PrEP exposure through the child’s 5th birthday. Our overall goal is to define a comprehensive safety profile of prenatal/postpartum PrEP in a unique ongoing cluster-RCT in Kenya that compares PrEP delivery approaches in >4,000 pregnant women. By leveraging existing PrIMA infrastructure, extending follow up from 9 to 60 months, and collecting new infant outcome data in this unprecedented cohort, we are uniquely positioned to assess infant PrEP exposure and outcomes.
Sponsor: National Institute of Health (5R01HD100201)
Award Years: 09/24/2019 – 06/30/2024
Principal Investigator: Jillian Pintye
Co-Investigators: Jared Baeten, Ruanne Barnabas, Sarah Benki-Nugent, Monica Ghandi (UCSF), David Gidden (UCSF), Renee Heffron, Grace John-Stewart, John Kinuthia
Staff: Julia Dettinger
Trainee: Micaela Haglund
PrEP Implementation for Young Women and Adolescents (PrIYA)
PrEP Implementation for Young Women and Adolescents (PrIYA) seeks to programmatically evaluate implementation, and determine best practices for providing PrEP to young women and adolescent girls in maternal-child health (MCH) and family planning (FP) clinics.
Sponsor: PEPFAR DREAMS/National Institute of Child Health and Human Development (5R01HD094630)
Award Years: 09/01/2017 – 06/30/2022
Principal Investigators: Grace John-Stewart, Pamela Kohler
Co-Investigators: Barbra Richardson, Jennifer Unger, Jillian Pintye, Jane Simoni
Trainees: Anna Larsen
Testing Implementation Strategies to Improve Delivery of PrEP for Pregnant and Postpartum Women in Kenya
Pregnancy is a high-risk time for acquiring HIV; pre-exposure prophylaxis (PrEP) is an effective, female-controlled, evidence-based intervention that is recommended during pregnancy in high-risk settings. Kenya is one of the few settings where PrEP is being systematically delivered in some regions during pregnancy. This project aims to determine what the barriers are to offering PrEP during routine pregnancy care clinics, test strategies to improve the delivery of PrEP in pregnancy care clinics, and quantify the budget impact of these strategies.
Sponsor: National Institute of Health (K01MH121124)
Award Years: 08/06/2019 – 07/31/2024
Principal Investigator: Anjuli Wagner
Mentors: Ruanne Barnabas (UW), Jared Baeten, Peter Cherutich (Kenya Ministry of Health), Grace John-Stewart, John Kinuthia, Kenneth Sherr, Bryan Weiner (UW)
PrEP Optimized for Mothers: Efficient PrEP Integration in MCH Clinics (PrEPARE)
PrEPARE seeks to identify ways to improve the efficiency of PrEP integration within MCH clinics through stakeholder engagement, identifying challenges to implementation in the health system, and piloting and evaluating optimized MCH-PrEP approaches that decrease health care workers’ workload and enhance client experiences.
Sponsor: National Institute of Health (R01HD094630)
Award Years: 09/25/2019 – 06/30/2020
Principal Investigators: Grace John-Stewart, Pamela Kohler
Co-Investigators: Kristin Beima-Sofie, Jillian Pintye, Anjuli Wagner
Staff: Julia Dettinger, Laurén Gomez
Scaling up integrated PrEP delivery in Kenyan maternal and child health clinics for pregnant and postpartum women (PrEP Scale)
This implementation science R01 study aims to test a scale-up package to expand the delivery of pre-exposure prophylaxis (PrEP) for pregnant and postpartum women in Kenya.
Sponsor: National Institute of Mental Health (R01MH135730)
Award Years: 01/01/2024 – 10/31/2028
Principal Investigators: Anjuli Wagner, John Kinuthia
Co-Investigators: Julia Dettinger, Brian Flaherty, Grace John-Stewart, Arianna Means, Felix Abuna, Nancy Ngumbau
mWACh-PrEP: A SMS-based Support Intervention to Enhance PrEP Adherence during Pregnancy and Breastfeeding
We propose a randomized trial to determine the effect of the mWACh-PrEP tool on PrEP adherence during pregnancy through the postpartum period. We will also gather data on cost and delivery using the Proctor Implementation Outcomes Framework to expedite translation into routine practice. Our overarching hypothesis is that mWACh-PrEP will improve PrEP adherence among mothers at-risk for HIV, be acceptable to patients and providers, and be cost-effective.
Sponsor: National Institute of Nursing Research (5R05NR019220)
Award Years: 09/18/2020 – 06/30/2025
Principal Investigators: Jillian Pintye, John-Kinuthia
Preventing, Detecting, and Treating HIV and Co-Infections
CMV Viremia and Mortality in Hospitalized HIV-infected Children (PUSH-CMV)
This study involves the use of data and archived specimens from the completed RCT Pediatric Urgent Start of HAART (PUSH), Grant: 5R01HD023412-25; PI: John-Stewart. This study aims to determine the impact of cytomegalovirus (CMV) viremia on mortality and duration of hospital stay, response to ART initiation, and immune activation and inflammation among children diagnosed with HIV infections while critically ill.
Sponsor: National Institutes of Health (2R21HD089821)
Award Years: 08/15/2018 – 07/31/2020
Principal Investigator: Jennifer Slyker
Co-Investigators: Grace John-Stewart, Irene Njuguna, Dalton Wamalwa
Staff: Laurén Gomez
Epstein-Barr Virus Replication, Malaria and Clinical Outcomes in Hospitalized HIV-Infected Children (PUSH-EBV)
Epstein-Barr Virus (EBV), reactivates frequently in hospitalized HIV-infected children, but its significance is unknown. Sub-microscopic malaria parasitemia is also common in many regions of the HIV epidemic and could contribute to EBV reactivation, mortality, and immune recovery. The proposed study will utilize blood specimens and data collected as part of a recently-completed clinical trial in Kenya to evaluate the frequency, correlates, and clinical significance of EBV viremia and malaria parasitemia.
Sponsor: National Institutes of Health (2R21HD102825)
Award Years: 04/01/2020 – 03/31/2022
Principal Investigator: Jennifer Slyker
Co-Investigators: Grace John-Stewart, Irene Njuguna, Barbra Richardson, Dalton Wamalwa
Viral Determinants of Natural Human Cytomegalovirus Transmission (CIHR-CMV)
This study follows a cohort of 100 women and their children, and performs CMV qPCR of saliva, urine, breast milk, and blood samples, to identify >50 transmission events. We will then use whole viral genome NGS to definitively determine the source of each CMV transmission, and genotype T/F viruses. We will then use mathematical modeling to define the probability of transmission based on the viral load and genotypes of a given exposure.
Sponsor: Canadian Institutes of Health Research (20R11969)
Award Years: 04/01/2018 – 03/31/2019
Principal Investigator: Jennifer Slyker
Maternal-Infant Virome Transmission: The Role of HIV and Antiretroviral Therapy (Virome MTCV)
The Virome MTCV study is a prospective cohort study of both HIV-infected and -uninfected women and their infants. This study will characterize the determinants of mother-to-child virome transmission and how components of the virome affect infant health. There will be a special emphasis on cytomegalovirus (CMV) due to its prevalence and known importance for human health.
This award also includes a protocol component, which involves testing archived breast milk samples from past studies to evaluate how HIV disease progression and ART use impact the composition of the maternal virome in breast milk.
Sponsor: Fred Hutchinson Cancer Research Center (0000966758)
Award Period: 09/06/2017 – 06/30/2019
Principal Investigators: Jennifer Slyker, Dalton Wamalwa
Co-Investigators: Bhavna Chohan, Grace John-Stewart, Barbra Richardson, Dalton Wamalwa
Staff: Emily Begnel
Planning grant for CMV suppression to reduce mortality in hospitalized, HIV-exposed children (CMV ICU)
The proposed planning grant will support activities to finalize the study design, develop the study protocol and all instruments, prepare the sites for implementation of the study, and convene key stakeholders to support execution and dissemination of the trial
Sponsor: National Institute of Allergy and Infectious Diseases (R34AI174980)
Award Years: 09/01/2023 – 08/31/2024
Principal Investigators: Jennifer Slyker, Dalton Wamalwa
Co-Investigators: Bhavna Chohan, Barbra Richardson, John Kinuthia
Development of a point-of-care wearable device for congenital cytomegalovirus screening in newborns (CMV WATCH)
Globally there is an increasing number of HIV-exposed but uninfected children and adolescents (HEU). We propose to evaluate HEU in Kenya, spanning from infancy to adolescence using different epidemiologic approaches to determine whether HEU have increased risk of adverse neurodevelopmental or mental health outcomes. We plan to screen a large population of HEU nationally and work collaboratively with stakeholders to review this data to inform approaches to screen, identify, and refer HEU with adverse outcomes, that could be used programmatically.
Sponsor: Thrasher Research Fund
Award Years: 01/01/2023 – 12/31/2024
Principal Investigator: Nuttada Panpradist
Mentors: Grace John-Stewart, Jennifer Slyker, Karl Bohringer
Identifying critical determinants of vaccine-induced cellular and humoral immunity from birth through childhood in HIV-exposed and unexposed children (INTEND)
The proposed studies offer an unprecedented opportunity to understand how HIV, co-infections, malnutrition and inflammation alter early life and long-term immune reactivity in African children. Results from this study have great potential to optimize vaccination strategies for children in areas of HIV prevalence, CMV and malnutrition.
Sponsor: National Institute of Allergy and Infectious Diseases (R01AI181634)
Award Years: 01/01/2023 – 12/31/2024
Principal Investigator: Jennifer Slyker, Cheryl Day (Emory University), Dara Lehmen (Fred Hutch)
Co-Investigators: Christopher Scharer (Emory)
Pediatric HIV reservoir determinants and consequences (PED HIV)
We have had 4 cycles of a renewed NIH R01 (NICHD R01-023412). Cycle 3 of the R01 involved a cohort of early-treated Kenyan infants (OPH cohort) which we have continued to follow-up to examine HIV reservoir. Our team has optimized a novel intact proviral DNA assay (IPDA) for Kenyan subtypes which provides quantitative estimates of intact and defective virus. Building on this work, we propose a competitive renewal of our R01 to examine the influence of cumulative and episodic CMV/EBV activation on the intact HIV reservoir and the relationship between HIV reservoir and long-term neurocognitive outcomes.
Sponsor: National Institute of Health (R01HD023412)
Award Years: 06/01/2022 – 04/30/2027
Principal Investigator: Grace John-Stewart, Dalton Wamalwa
Project Directors: Sarah Benki-Nugent, Jennifer Slyker
Co-Investigators: Barbra Richardson, Stephen De Rosa
Assessing Vaginal Microbial Communities as a Risk Factor for HIV Acquisition in Pregnant and Postpartum Kenyan Women
Sponsor: National Institutes of Health (1F32HD100202)
Award Period: 11/01/2019 – 10/31/2020
Candidate: Erica Lokken
Sponsors: Grace John-Stewart, Scott McClelland (UW)
Mentors: David Fredricks (UW Medicine), John Kinuthia (Kenyatta National Hospital), Tim Randolph (Fred Hutch)
In this proposal, the candidate will first perform hierarchical clustering analysis utilizing species-specific quantitative PCR data from our existing HIV acquisition dataset to generate vaginal bacterial profiles describing distinct groups of women based on the collective concentrations of 20 bacterial taxa, including the minority species that have been most closely associated with HIV acquisition. The candidate will then examine the association between the distinct bacterial profiles and HIV acquisition.
Preventing Mtb Infection in HIV-Exposed Infants (iTIPS)
This randomized controlled trial aims to determine if isoniazid preventative therapy (IPT) prevents primary Mycobacterium tuberculosis (Mtb) infection in HIV-exposed uninfected infants in Kenya.
Sponsor: Thrasher Research Fund
Award Years: 09/01/2015 – 08/31/2019
Principal Investigators: Grace John-Stewart, John Kinuthia
Co-Investigators: Tom Hawn (DAID, UW), Sylvia LaCourse (DAID, UW), Barbra Richardson (Biostatistics and Global Health, UW), Elizabeth Maleche-Obimbo (UoN), Lisa Cranmer (Emory University), Daniel Matemo (KNH)
Trainee: Danae Black
Click here to view the iTIPS Study Protocol.
Click here to view the iTIPS Statistical Analysis Plan.
Click here to view the iTIPS primary trial publication.
Click here to view the iTIPS 24-month observational publication and supplemental data.
Impact of Maternal HIV on Mtb Infection Among Peripartum Women and Their Infants (MITIPS)
Prospective observational cohort study enrolling HIV-infected and uninfected pregnant women and their infants to determine the (Aim1) effect of maternal HIV on risk and timing of maternal peripartum Mtb infection, (Aim 2) influence of maternal HIV on risk of infant Mtb infection, and (Aim 3) effect of maternal HIV status and peripartum stage on LTBI test performance, including interferon gamma-release assays (IGRA) and tuberculin skin tests (TST).
Sponsor: National Institute of Allergy and Infectious Diseases (NIAID) (2K23AI120793-01A1)
Award Years: 04/2019 – 03/2021
Principal Investigator: Sylvia LaCourse
Mentors: Grace John-Stewart, Amita Gupta (Johns Hopkins)
Click here to view the study protocol.
Effect of HIV Exposure and Infection on Immunity to TB in Children (PEDS TB)
The proposed study will utilize specimens from unique cohorts of HIV-infected, HEU, and HIV-unexposed (HUU) children to determine predictors of anti-mycobacterial immunity (including HIV exposure, HIV infection, antiretroviral treatment, and other factors), the influence of HIV exposure or infection on ‘trained immunity’, and the effects of this immunity on susceptibility to TB.
This study uses data from the Impact of maternal HIV on Mycobacterium tuberculosis infection among peripartum women and their infants (MITIPS) study (PI: LaCourse).
Sponsor: National Institutes of Health (05R01AI142647)
Award Years: 01/01/2019 – 12/31/2023
Principal Investigator: Grace John-Stewart, Cheryl Day (Emory University)
Site Principal Investigators: Elizabeth Maleche-Obimbo (University of Nairobi), Dalton Wamalwa (Kenyatta National Hospital)
Co-Investigators: Sylvia LaCourse, Jennifer Slyker, Barbra Richardson
Staff: Jaclyn Escudero
Click here to read the study protocol.
NanoDisc0MS Measured Mtb Antigen Peptides for TB Diagnosis and Treatment Monitoring in HIV-Infected Children
The goal of this study is to evaluate the performance of a novel blood-based diagnostic (NanoDisk-MS) to detect M. tuberculosis antigens for TB diagnosis (Aim 1), mortality prognosis (Aim 2), and treatment response in HIV-infected children (Aim 3).
Sponsor: National Institute of Allergy and Infectious Diseases (1R21AI143341)
Award Years: 03/2019 – 03/2021
Principal Investigator: Sylvia LaCourse
Mentors: Grace John-Stewart (UW), Amita Gupta (Johns Hopkins)
Click here to view the study protocol.
Dynamics of TB Immune Response in Peripartum HIV-Infected and HIV-Uninfected Women
The goal of this project is to characterize the effect of pregnancy on innate and adaptive immune responses to Mtb using stored samples that permit the study of immune responses pre-, during, and post-pregnancy.
Sponsor: National Institute of Child Health and Human Development (R21HD098746)
Award Years: 04/2019 – 03/2021
Principal Investigators: Sylvia LaCourse, Javeed Shah (UW)
Air Pollution Exposures in Early Life and Brain Development in Children (ABC)
The award will support the establishment of a well-characterized prospective birth cohort for supporting studies on environmental exposures and child neurodevelopment in Kenya. We will characterize early life air pollutant exposures in a densely populated urban environment (Nairobi) and determine whether children with higher air pollutant exposures in early life have worse neurodevelopmental outcomes. We will develop a novel hybrid approach to modeling air pollution exposure, involving combined indoor and mobile air monitoring.
Sponsor: National Institute of Environmental Health Sciences (5R01ES032153)
Award Years: 09/01/2020 – 08/31/2025
Principal Investigator: Sarah Benki-Nugent
Co-Investigators: Catherine Carr, Brent Collett, Edmund Seto, Timothy Larsen, Julian Marshall, Scott McClelland, Barbra Richardson, Anne Riederer, Chris Simpson (UW); Michael Gatari, Elizabeth Maleche Obimbo, Beatrice Mutai, Faridah Were (University of Nairobi); Susan Wamithi (Aga Khan University; Micahel Willoughby (RTI)
HEU outcomes: population-evaluation and screening strategies (HOPE)
Globally there is an increasing number of HIV-exposed but uninfected children and adolescents (HEU). We propose to evaluate HEU in Kenya, spanning from infancy to adolescence using different epidemiologic approaches to determine whether HEU have increased risk of adverse neurodevelopmental or mental health outcomes. We plan to screen a large population of HEU nationally and work collaboratively with stakeholders to review this data to inform approaches to screen, identify, and refer HEU with adverse outcomes, that could be used programmatically.
Sponsor: National Institute of Child Health & Human Development (5R61HD103079)
Award Years: 09/01/2020 – 08/31/2025
Principal Investigators: Grace John-Stewart, Irene Njuguna
Co-Investigators: Sarah Benki-Nugent, Shannon Dorsey, Manasi Kumar, Christine McGrath, Anjuli Wagner, Dalton Wamalwa
Click here to read the study protocol.
COVID-19 Affecting Women, Children, and Families
Understanding and Addressing Barriers to COVID-19 Testing in the Somali Community in King County, WA: A Community-Driven Strategy
Sponsor: University of Washington
Award Years: 2020-2021
Principal Investigators: Keshet Ronen, Ahmed Ali (Somali Health Board)
Preliminary data from the Somali Health Board (SHB), a Somali-led grassroots organization, suggest the King County Somali community experiences barriers to timely COVID-19 testing through existing testing channels at community health clinics. In response, SHB has arranged a series of testing fairs in South King County, where the Somali community is concentrated, partnering with religious and community leaders to mobilize turnout. Attendance at testing fairs to date has been high, underscoring substantial unmet need for testing in this community, and presenting an opportunity to understand community testing needs and develop a sustainable strategy to meet them.
Leveraging SHB’s deep community roots and planned testing fairs, our mixed-methods, community-driven proposal seeks to (1) quantitatively determine prevalence and correlates of access to timely COVID-19 testing in the King County Somali community; and (2) gather perspectives of multiple stakeholders (community members, healthcare workers, and policymakers) on community testing needs and how they may be integrated into King County’s testing strategy.
This project will result in a deeper understanding of facilitators and barriers of COVID-19 testing in the King County Somali community, and actionable recommendations to support testing in this heavily impacted community, improve health equity, and support local epidemic control.
COVID-19 Prevalence, Household Transmission, and Antibody Response Among Pregnant Women and Their Pediatric and Adult Household Members in South King County, WA State
Sponsor: Center for Disease Control and Prevention
Award Years: 2020-2021
Principal Investigators: Alison Drake, Sylvia LaCourse
Co-Investigators: Alisa Kachikis (OB/GYN), Janet Englund (Pediatrics), Alex Greninger (Lab Medicine)
The University of Washington is conducting a study on COVID-19 prevalence, household transmission, and antibody response among women who are pregnant and their pediatric and adult household members in South King County, Washington. The study will investigate adverse health outcomes and other factors associated with symptomatic and asymptomatic SARS-CoV-2 infection among 1,000 pregnant women. It will also estimate potential household transmission and durability of antibodies (SARS-CoV-2 specific IgG) over time (up to 6 months) among pregnant women and their pediatric and adult household contacts.
