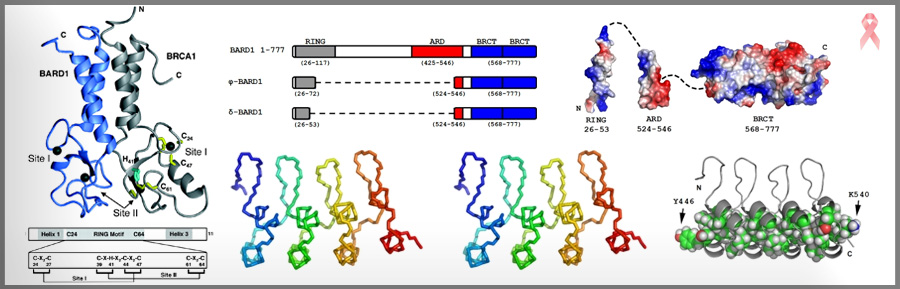
BRCA1/BARD1 is a large heterodimeric protein that is implicated in inherited forms of breast and ovarian cancer. Our current studies focus on the N-terminal “RING” domain where many inherited missense mutations occur. The RING domain is responsible for BRCA1/BARD1’s only known biochemical function—it acts as a ubiquitin E3 ligase. Questions of interest include: How does binding of BRCA1/BARD1 to an E2 activate transfer of ubiquitin? and What are the cellular protein targets of BRCA1-dependent ubiquitination?
We are also focusing on the BARD1 subunit, as little is understood regarding its function. We have solved the crystal structure of the ankyrin repeat domains of BARD1 and are looking for binding partners.
Small Heat-shock Proteins – First responders during cellular stress
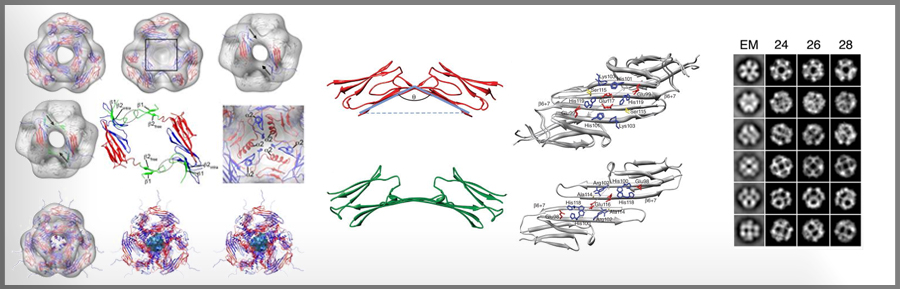
aB -crystallin (aB) and HSP27 are two of the ten human sHSPs, a class of molecular chaperones known as “holdases” for their ability to maintain the solubility of partly unfolded proteins that arise under conditions of cellular stress. Due to their tendency to form large, dynamic oligomeric structures, sHSPs are refractory to x-ray crystallography. We are using a combination of solution-state and solid-state NMR, SAXS, EM, and tandem MS/MS to define the structures and dynamic properties of aB and Hsp27. Although a complete understanding of the cellular functions of aB and HSP27 is not yet available, the proteins are implicated in certain inherited forms of dystrophies as well as in breast and other cancers. Our current studies aim to define the mechanisms by which cellular conditions activate sHSP function and to identify the ways in which sHSPs recognize and bind partly unfolded proteins.
Protein Ubiquitination – How is it accomplished?
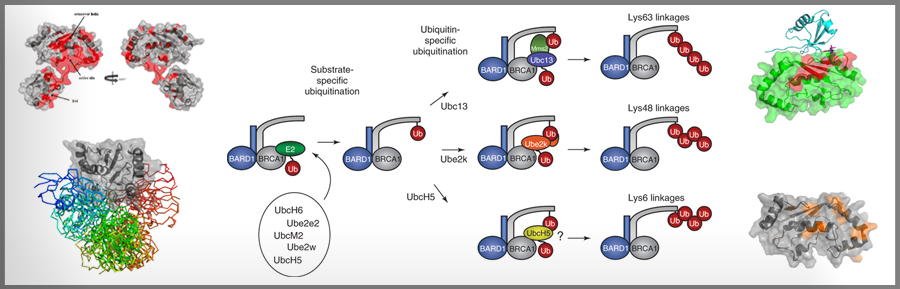
Although the Nobel Prize in Chemistry was awarded for the discovery of ubiquitin in 2004, our understanding of how the small protein, ubiquitin, is transferred covalently to a protein target still has some fundamental holes. We are focusing on mechanistic questions such as How does binding of an E3 activate an E2~Ub to transfer its ubiquitin? and How do RING-Between- RING E3s (such as parkin) differ in their function from conventional RING E3s (such as BRCA1/BARD1)? Towards this aim, we are using a variety of structural approaches to study the highly dynamic E2~Ub species and the very transient complex formed by an E3/E2~Ub that is poised for reaction.




