 |
|||||||||
|
Research Feature Stories: Other News: |
Small Differences in Genetic Code Affect Human Functions
According to Associate Professor of Genome Sciences Deborah Nickerson, “As our knowledge of the baseline reference sequence increases, so will our need to define the level of natural variation in human populations. Basic sequences are 99.9 percent similar, but that tenth of a percent variation may hold the key to our understanding of a number of diseases.”
|
Laboratory researchers studying how genetic variations affect an individual’s susceptibility or resistance to disease are themselves from diverse populations. |
 |
Nickerson directs a $10 million grant from the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) to study genetic variation. Awarded to the UW and the Fred Hutchinson Cancer Research Center, the grant is one of 11 similar NIH-financed programs across the country. The goal is to apply and expand Human Genome Project data to learn how small differences in the genetic code sequence affect human functions, especially susceptibility and resistance to disease.
“We’re interested in applying sequencing technology to human genes known to be involved in disease,” said Nickerson. For example, there are candidate lipid metabolism genes involved in cholesterol processing and transport. Some people get heart disease early in life, and genetic variation could possibly identify individuals with that susceptibility.
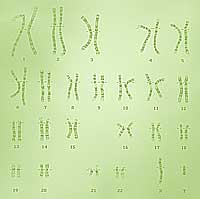 |
A karyotype reveals the size, shape and number of a set of chromosomes. |
The UW-Fred Hutchinson Center team is studying lung and blood diseases—like blood clotting abnormalities and hardening of the arteries, for example—with a concentration on inflammation. Many genes take part in the body’s inflammatory response. Specific lung diseases can sometimes trigger a prolonged inflammation. Genetic variation might help researchers understand why this occurs in some individuals and not others. Collaborators at the Fred Hutchinson Center will develop the statistical methods for identifying at-risk individuals.
Another of Nickerson’s research interests is pharmacogenomics—how variation studies might help identify the most effective drugs for a particular individual. One of her projects is examining pharmaceuticals for high blood pressure. There are, for example, more than 100 drugs on the market to treat high blood pressure, but choosing the right one is often a matter of trial and error. Nickerson said that understanding variation could help physicians prescribe the best drug from the start.
|
| UW AMC Medical Center | UW School of Medicine | Harborview | UW MC | Search UW AMC | UW Home | Contact Us | ©2001-2002, University of Washington Academic Medical Center. All rights reserved. Please honor our copyrights. |
|