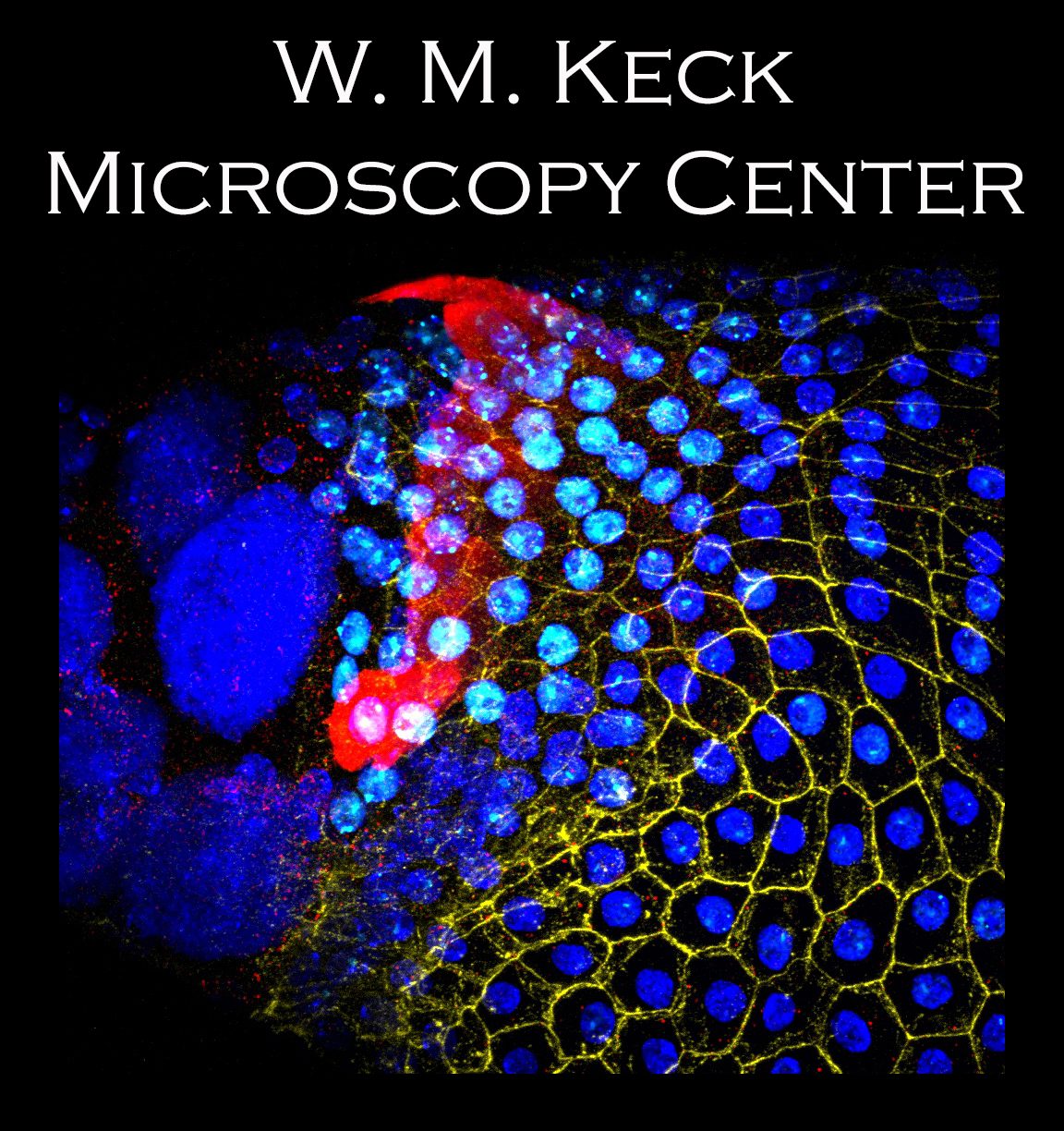
Successful microscopy depends on proper sample preparation, so start with a solid foundation!
If you have any questions about sample preparation, contact the Keck Center Manager.
Microscope Coverslips (Click here to learn more!)
Coverslip Selection
MICROSCOPE OBJECTIVES ARE, BY DESIGN, CORRECTED FOR #1.5 (170um) GLASS COVERSLIPS. Some objectives can correct for other thicknesses (e.g., some water immersion objectives), but most cannot. #1.0 (120um) COVERSLIPS (OR OTHER THICKNESSES) SHOULD TYPICALLY NOT BE USED.
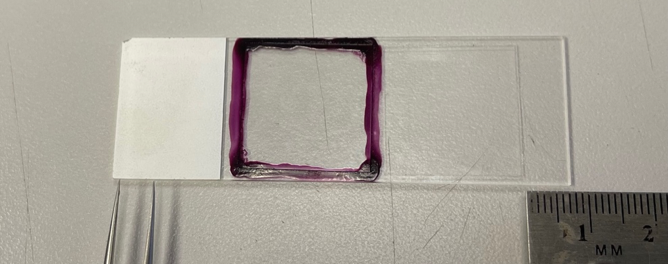
Coverslip Placement on the Microscope Slide
On inverted microscopes, such as the microscopes available at the Keck Center, coverslip placement on the microscope slide is VERY important. Since the microscope slide is “upside down” on an inverted microscope (i.e., coverslip faces down towards the objective), the ends of the microscope slide that rest on the stage’s slide holder must not be covered by the coverslip (or by stickers added to the label end of the slide). If the coverslip extends to the edge of the slide, it will tilt the slide and either complicate, or even prevent, successful imaging. Square coverslips should be positioned in the middle of the slide. For rectangular coverslips, at least 5-7mm of slide should be exposed at both ends of the slide for optimal imaging. Refer to the image below for a visual representation:
Mounting Media
Many different mounting media (i.e., the liquid surrounding the specimen between the microscope slide and the coverslip) can be used for mounting specimens for microscopy. The simplest mounting medium is an 80% glycerol in water combination, but this has no anti-fade properties for preserving fluorophore/dye fluorescence. An antifade compound such as DABCO can be added to an 80% glycerol in water stock for a “homemade” anti-fade mounting medium. Commercially available liquid mounting media, such as Fluoromount, Vectashield, or Prolong can be purchased. Prolong Gold, for example, is one of the most popular mounting media for Keck Center users.
Some commercially available mounting media remain liquid, and some harden/set into a solid resin. Liquid mounting media typically require sealing of the coverslip with nail polish; the corners of the coverslip should be tacked down first, then after those tacks are dry the rest of the coverslip edge can be sealed without the coverslip shifting. One advantage of hardening mounting media is that sealing of the coverslip is not necessary; in fact, sealing the coverslip will prevent curing. DO NOT SEAL THE COVERSLIP IF YOU ARE USING A HARDENING MOUNTING MEDIUM! Importantly, the refractive index of the mounting medium changes during curing, so samples in hardening mounting media must be cured for at least 24 hours before imaging.
Some mounting media are available with DAPI, but these are not recommended. The concentration of DAPI is typically far too high, and can cause excessive background and/or bleed-through. Some claim that DAPI only fluoresces when bound to DNA, but this is false. Excess, unbound DAPI will fluoresce, and it will cause background. DAPI staining should be incorporated into the staining protocol, should be brief, and should be thoroughly washed out before mounting.
Specimen Mounting
- Tissue sections, for the most part, should be mounted directly on #1.5 coverslips after sectioning, not on a thick slide. Whether you mount your sections this way or the traditional way (directly onto a 1″ x 3″ slide, then topped with a coverslip), you should take care when adding mounting medium so that there are no bubbles and the layer is uniform.
- Cultured cells should be grown directly on acid-washed #1.5 coverslips, then mounted on a slide using a suitable mounting medium. This is an example of a protocol for growing adherent cells on coverslips for immunofluorescence microscopy.
- Petri dishes and multi-well chambered coverslips/plates must have #1.5 coverglass bottoms for any confocal or high-resolution widefield imaging. Here are some companies that supply these sample containers:
- Nunc Lab-Tek II Chambered Coverglass (note that these are NOT the same as Nunc Chamber slides, which are incompatible with live cell imaging).
Live cell (or organism) imaging poses many challenges, one of them being high background fluorescence (especially in the green part of the spectrum), due to components in the surrounding media. Phototoxicity and bleaching can also be problematic. To avoid these issues you may want to use media specifically designed to protect cells and reduce background fluorescence during long periods of light exposure.
Stains and Dyes: Before you prepare your samples, make sure the dyes you have chosen are compatible with the microscope you plan to use! Check with the Keck Center Manager if you have questions!