The goal of research in the Miller Lab is to understand fundamental mechanisms that allow animals to maintain homeostasis and survive in changing conditions.
The environment we live in is constantly changing. Temperature, food, toxins, predators, and even oxygen can fluctuate rapidly. In order to survive, animals must constantly monitor environmental conditions and adjust physiological function accordingly. Understanding how animals maintain homeostasis could help us to devise new strategies to treat a whole host of human pathologies, such as diabetes, cancer, neurodegenerative diseases, stroke, and traumatic injury.
Ongoing projects in the lab focus on advancing our understanding of fundamental aspects of homeostasis in animals. We focus on two main environmental factors: oxygen (O2) and hydrogen sulfide (H2S). Check out our publications; or, if you want to search for us on PubMed, click here.
OXYGEN
O2 is essential for all animals (with one notable exception!). Decreased O2 availability (hypoxia) is associated with cellular death and damage in stroke, heart attach, severe blood loss, and ischemia/reperfusion injury. Inappropriate activation of hypoxia response pathways has also been implicated in diabetes and cancer. We are working to map out novel hypoxia response pathways and how they integrate with normal cellular functions.
Hypoxia and protein homeostasis.
We have found that specific hypoxic conditions disrupt protein homeostasis in C. elegans. Protein homeostasis is a coordinated network of cellular processes that works to ensure cellular proteins remain folded and functional. Defects in protein homeostasis are associated with devastating neurodegenerative diseases such as Huntington’s Disease, Parkinson’s Disease, and Alzheimer’s Disease. We want to know how protein homeostasis is disrupted in hypoxia – what coordinates this change in cellular physiology, and what parts of the protein homeostasis network are affected?
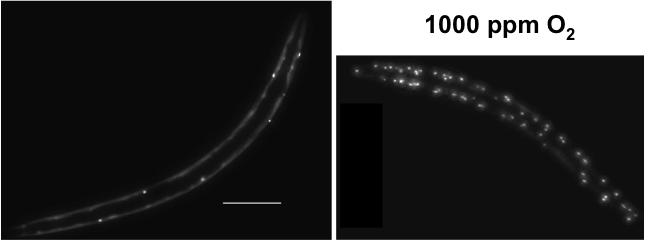 These worms express yellow fluorescent protein (YFP) fused to a series of polyglutamine residues. In hypoxia the number of fluorescent foci (aggregates) dramatically increases. Fawcett et al., Aging Cell 2015.
These worms express yellow fluorescent protein (YFP) fused to a series of polyglutamine residues. In hypoxia the number of fluorescent foci (aggregates) dramatically increases. Fawcett et al., Aging Cell 2015.
Hypoxia and developmental control (suspended animation).
In the complete absence of O2 (anoxia), C. elegans enters into a state of suspended animation in which all cell division and microscopically observable life processes halt. Upon return to O2 (even after 24 h!), the animals re-animate as if nothing happened. We have discovered that when there is just a little bit of O2 (1000 ppm, or 0.1%), worms do not enter into suspended animation. Instead, these animals enter into a hypoxia-induced diapause where the animals continue to move but post-embryonic development and reproductive functions reversibly arrest. We are working to understand how the cell division cycle is arrested by hypoxia responses, how developmental processes across the entire animal are coordinately stopped and then restarted without error, and what the molecular pathways that mediate this response are. This is a picture of the same worm before and after exposure to hypoxia. Arrows mark embryos that enter into hypoxia-induced diapause. Miller and Roth, Current Biology 2009.
This is a picture of the same worm before and after exposure to hypoxia. Arrows mark embryos that enter into hypoxia-induced diapause. Miller and Roth, Current Biology 2009.
HYDROGEN SULFIDE
H2S is recognizable to almost everyone – it is what gives rotten eggs their nasty smell! H2S is common in the environment because of volcanoes (Yellowstone National Park, Lechugilla caves) and as a result of anaerobic bacteria (peat bogs, sewers). But, you may be surprised to learn that YOU are constantly producing H2S in your cells right now! H2S has been shown to have important roles in cellular signaling, neuromodulation, and regulating vascular tone. You might be even MORE surprised that treatment with H2S can improve outcome in animal models of hypoxia and ischemia/reperfusion injury.
Ongoing projects in the lab are aimed at understanding the difference between “beneficial” and “toxic” exposure to H2S, to define the cellular and organismal processes that mediate normal and pathological responses to H2S.
Epigenetic effects of exposure to H2S.
We have discovered that exposure to H2S leads to epigenetic changes that help animals respond better if they encounter H2S again. We are working to figure the mechanistic basis of this “epigenetic bookmark”.
Factors that mediate responses to H2S.
At low concentrations, H2S increases lifespan and stress resistance in worms. However, at high concentrations the animals become paralyzed and then die. We have taken unbiased genetic and proteomic approaches to begin to define the differences between exposure to low and high concentration H2S, and we are working to understand not only the response to H2S but how this response changes how cells (and organisms) respond to other changes, such as decreased food availability or hypoxia.
