Bladder Surveillance
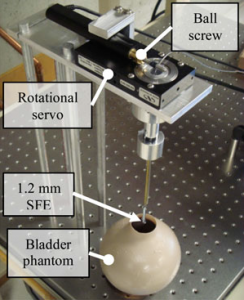
Components of automated steering apparatus for controlling the trajectory of an ultrathin endoscope for fully automated bladder surveillance (Burkhardt, et al).
Due to the high recurrence rate of bladder cancer, frequent cystoscopic surveillance is required for patients following initial diagnosis. During examination, the urologist manipulates a cystoscope to thoroughly inspect the bladder wall. These procedures constitute a significant percentage of urologists’ work-load, making bladder cancer the most expensive cancer to treat over the patient’s lifetime [1].
The gold standard examination of the lower urinary tract consists of an optical examination of the bladder urothelium, the tissue layer lining the interior of the urinary tract, or cystoscopy. Long-term endoscopic follow-up in the bladder after treatment is crucial to guarantee early detection and treatment of cancer recurrence. However, while cystoscopy is the “gold standard” for a routine surveillance exam, it remains imperfect with various experimental studies demonstrating 4-10% missed bladder tumors by conventional cystoscopy [2-4]. To address these challenges research at the HPL has focused on the following developments:
Development of an automated mechanism for bladder surveillance
- Active programmable remote steering mechanism and efficient motion sequence for bladder cancer detection and postoperative surveilllance.
- Reduced workload and cost of cystoscopy by relieving the urologist from performing surveillance by controlling the motion of a miniature flexible endoscope using image-based feedback.
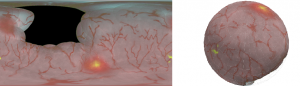
Reconstruction of bladder surface mosaics from endoscopic video
- Frames selected from cystoscopy using the SFE, are stitched into a mosaic and mapped to a reconstructed surface creating a 3-D surface model of the bladder that can be expediently reviewed.
Multimodal flexible cystoscopy for creating co-registered panoramas of the bladder
- The SFE is able to acquire wide-field multimodal video from a bladder phantom with fluorescence cancer “hot-spots”.
Acknowledgments
Funding for the fabrication of the multimodal SFE instrumentation provided by NIH grants (R01EB008119, RC1EB010900 and pilot project from U54CA136429). Funding for the software development was provided by the UW Center for Commercialization and the W.F. Coulter Foundation administered through UW Bioengineering. Funding for the development of an automated steering mechanism was provided by NIH/NCI grant CA094303.