Cystic Fibrosis Research Development Program
The CFF RDP has been in existence at the University of Washington and the Seattle Children’s Research Institute (SCRI) since 1989. Our center’s three-decade history of promoting basic, clinical and translational CF research originated from the RDP, and the RDP remains its foundation. The scientific and human resources of the RDP have given rise to the Therapeutics Development Network (TDN) Coordinating Center (Goss/Hamblett PI’s), TDN pediatric and adult clinical sites (Gibson and Aitken PI’s), and two CF NIH P30 grants including the current Translational Research Center to Expedite Novel Therapies in Cystic Fibrosis (Ramsey/Greenberg PIs). Our RDP has generated new knowledge about CF infections, lung and intestinal disease, and the basic biology of CF pathogens. The RDP has developed new therapeutics in use world wide, and has fostered collaboration within our institution, and internationally. The RDP has trained several generations of CF researchers and clinicians, including innovative physician-scientists committed to CF research over the duration of their careers, and leaders in CF research and care at other institutions. The RDP also developed internationally-valued resources such as isolate collections, mutant libraries, genomics resources, and research tools utilized by hundreds of investigators worldwide.
Cystic Fibrosis Research Translation Center
Since its inception in 2010, the UW CFRTC has been closely linked to the development of the new Cystic Fibrosis Transmembrane Regulator (CFTR) modulators. The Clinical Core is an integral part of the CF Foundation (CFF) supported Therapeutics Development Network (CF-TDN) Coordinating Center. As such, multiple UW CFRTC members have served as key advisors to both the CF Foundation and Vertex in design of the pre-clinical and clinical trials for development of 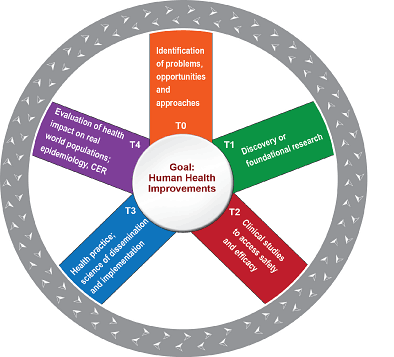 ivacaftor and correctors such as VX809 and VX-661. Since the approval of ivacaftor, the UW CFRTC has also been closely involved in 3 critical observational studies to decipher both the early physiologic and microbiologic changes that occur with initial ivacaftor administration (Acute Response to Ivacaftor Study in CF – the ARIS in CF) and more long term changes (US GOAL study and GOAL-E2).
ivacaftor and correctors such as VX809 and VX-661. Since the approval of ivacaftor, the UW CFRTC has also been closely involved in 3 critical observational studies to decipher both the early physiologic and microbiologic changes that occur with initial ivacaftor administration (Acute Response to Ivacaftor Study in CF – the ARIS in CF) and more long term changes (US GOAL study and GOAL-E2).
The ivacaftor experience serves as a paradigm for successful and iterative translational (T) research (Figure 1: Translational Wheel). A small molecule profoundly changes the health of CF patients, and by closely probing those changes in human pathophysiology, we are developing better biologic and clinical measures to further therapeutic development.
The UW CFRTC strategic vision is:
To continue to serve as a key international resource to catalyze the translation of data emerging from basic studies in digestive diseases, nutrition, metabolism, and infection to stimulate the development of new CF treatment approaches with special emphasis on development of CFTR modulators and how these drugs will impact in vivo epithelial physiology, cellular metabolism and microbiologic:host interactions.
We are proud that our members have funded research spanning all phases of translation from laboratory science (T0,T1) to therapeutic development (T2,T3) and epidemiology (T4).

