Franklin Faust, BS in Neurobiology, a research team member of UW Alzheimer's Disease Research Center, reports back with summaries of the neuroscience talks presented in Day 1 of Exploring Frontiers: Predicting Biology hosted by the Allen Institute for Brain Science. The July 25th afternoon sessions featured brain researchers from around the world. They discussed the use of modeling to better understand the brain.
The Allen Institute for Brain Science is a collaborator of the UW Alzheimer's Disease Research Center, on such projects such as the Aging, Dementia and Traumatic Brain Injury Study.
Dr. Peter Robinson, PhD, University of Sydney, discussed how scientists develop their own specific models but need a broader picture in order to inter-relate these models effectively. Dr. Robinson suggests that multi-scale, integrative models across experimental modalities can yield better links between theory and experiment. He presented example applications investigating normal and epileptic brain activity, electroencephalography, evoked responses to stimuli, brain state tracking, and brain modes.
Dr. Gaute Einevoll, PhD, Norwegian University of Life Sciences, discussed the importance of building large-scale models, which measure both population and systems-level activity through multiple modalities. Einevoll suggests that this will help to bridge the gap in scales of activity and allow for systematic comparison and refinement of brain model, while also offering opportunities to falsify theories and advance the field of neuroscience. Einveoll used the Allen Institute’s model of mouse V1 cortex to make predictions of local field potentials, and he found that his candidate models produced different results than the raw data. Einevoll plans to include genetic variation in future models.
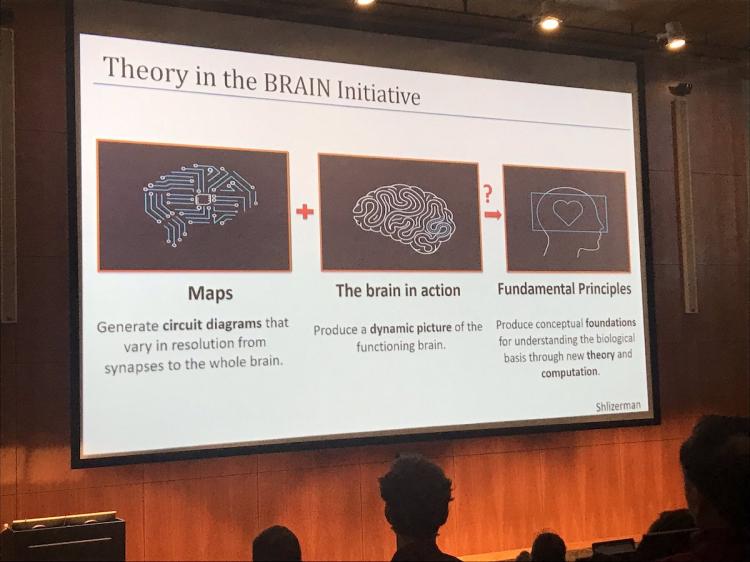
Dr. Adrienne Fairhall, PhD, University of Washington, reviewed the state of theory in neuroscience and emphasized its importance. Arguing that neuroscientists have different definitions of theory, Dr. Fairhall broke down theory into three categories: “Big T Theory”, data analysis, and modeling. Dr. Fairhall proposes that Theory is the heart of the BRAIN initiative and that the development of models is necessary to develop new theories for how we can understand the brain.
Dr. Anton Arkhipov, PhD, Allen Institute for Brain Science, presented on the Allen Institute’s V1 mouse models. These include biophysical and point-neuron multi-modal models which have been created for each specific cell, as well as data sets of connection probabilities, synaptic strength, axonal delays, and synaptic targeting of dendrites. Simulations have been compared to experimental data, yielding neuron connectivity and arrangement insights with regards to direction-selectivity. The Allen Institute created the Brain Modeling ToolKit software suite and SONATA modeling file format to support this project. These tools, the models, and simulation results are all freely available via the Allen Institute Modeling Portal.
Dr. Michael Reimann, PhD, l’École Polytechnique Fédérale de Lausanne, presented his work in neuronal connectivity, which applies micro-scale connectomics to the macro-scale mouse neocortex. AIB's voxelized connectivity model was used to project strength for each voxel, thereby offering higher resolution from region to region. However, the model requires outside data on connectivity patterns, so the Mouse Light dataset was leveraged to a break down how morphology connects to activity in the mouse brain. Dr. Reimann’s model “recreates biological trends that have been characterized on several scales, and it predicts that an established principle of a scale invariant structure of connectivity extends down to the level of individual neurons. It will enable biologically more detailed whole-brain simulations and can serve as a powerful null model for future discoveries in long-range micro-connectomics.” The model is available on the blue brain portal online under “mouse connectomics”.
Dr. Hiroki Ueda, MD, PhD, RIKEN Center, presented his team’s small-scale model’s findings that sleepiness and sleep/wake regulation is mediated by a handful of key proteins. Their Averaged Neuron Model simulated the electrophysiology of slow-wave sleep found in pyramidal neurons. Through bifurcation analysis, triple CRISPR methodology, and a combination of respiratory wave, EEG, and EMG experiments, they found results consistent with a hypothesis that phosphorylation-dependent regulation of the Ca2+-dependent hyperpolarization pathway underlies the regulation of sleep duration in mammals. They have also developed a Simplified Average Neuron model, which has given insights into the importance of K+ leak channels in non-REM sleep.
A panel discussion led by Christoph Koch, PhD, Chief Scientist and President of the Allen Institute for Brain Science, focused on the lack of emerging theory in neuroscience over recent decades. He said that in order to understand and cure neurodegenerative disease and psychiatric conditions, neuroscience needs to do a better job of combining data, small and large models, and theory in a comprehensive scientific framework. While the researchers disagreed on which aspects of this framework should be emphasized most, they agreed that all of these elements were important for pushing the field forward. —Franklin Faust
Related Stories
Dispatch from NeuroFutures2016: Dr. Anne Churchland: At the June 19th public lecture, a former UW post-doc, now leading experimental neuroscientist, talks about how she uses optogenetics to probe decision-making in the brain.
Tackling the Science of Brain Injury and Dementia Risk: With colleagues at the Allen Brain Institute and UW Medicine, the UW ADRC Neuropathology Core team created a publicly available databank resource for studying the later effects of TBI in the aging brain, called the Aging, Dementia, and TBI Study.