On May 9, Alzheimer's disease researchers from around the country gathered at the University of Washington for the MODEL-AD Symposium, a day of talks and discussion of current directions in the field and new ways of modeling risk and resilience in human and animal models of Alzheimer's disease. The event was co-hosted by Sage Bionetworks and the UW Alzheimer's Disease Research Center.
Among many other presentations, ADRC talks presented at the symposium spanned new human cell models of sporadic Alzheimer's disease, the search for protective genes, human resilience, and the newly recognized role of TDP-43 protein in dementia in older adults.
Neuropathology of AD Resilience Informs Development of In Vivo Models and Targets
At the MODEL-AD conference, a consensus emerged that it's past time look beyond amyloid beta to understand Alzheimer's disease and treatment targets, as no therapeutic aimed at amyloid has resulted in an effective treatment. Other pathologies often play a role in the disease. From the position of a neuropathologist, Caitlin Latimer, MD, PhD, faculty in UW Pathology, offered her ideas about the most fruitful disease targets and questions to inform the development of animal and human cell models and therapeutics. Specifically, Latimer thinks that the Alzheimer's field can learn important lessons by studying the brains of individuals who are either resistant or resilient to neuropathological change.
Resistance is a natural ability to avoid ever developing a certain type of pathology in the brain; Resilience is a brain's ability to cope and maintain cognitive function, despite having a load of brain pathology that would be expected to cause symptoms.
"The idea of studying resistant and resilient people is to try to determine the underlying mechanisms," said Latimer, "and to use those as a roadmap and to try to understand potential mechanisms to treat people who don't have natural mechanisms against the pathology."
She shared interesting findings that complicate the concept of resistance and resilience. For example, studies of neuropathological findings from both the UW Adult Changes in Thought Study and the Rush University's ADRC have found that being resilient was associated with the absence other types of pathology, such as vascular injury, TDP-43, or Lewy bodies. "There is an idea emerging that perhaps being resilient to Alzheimer's pathology of amyloid and tau is actually due to a resistance to developing other forms of pathology," said Latimer. Since then, she has provided more evidence for this possibility.
Latimer described her ADRC team's recent study using brain tissue resources from the Adult Changes in Thought study. She found a reason to recommend that researchers devote their time and resources to studying the protein called TDP-43. Her most striking finding was the fact that TDP-43 strongly associated with dementia: the brains of older people who were resilient or resistant did not have TDP-43 pathology, yet almost all of the brains from age-matched people with symptoms of dementia did have TDP-43 pathology. TDP-43 seems to put older people at high risk of dementia. The takeaway question from Dr. Latimer: "Is resilience in Alzheimer's disease really just resistance to TDP-43?
Latimer concluded that the field now needs to move on to modeling these proteotoxicity pathways in worms, mice, and human induced pluripotent stem cells models, in order to understand how TDP-43, tau, and amyloid are interacting or working in synergy to cause disease.
Insights from Human Stem Cell Models Suggest New Therapeutic Targets
Jessica Young, PhD, Assistant Professor, UW Pathology, uses induced pluripotent stem cells (IPSCs) to study the biology of Alzheimer's disease. To create IPSCs, researchers take skin cells of study participants, chemically reprogram them to the pluripotent state, and then differentiate the cells into neurons. This 'disease-in-a-dish' technology allows researchers to capture an individual human's genetic background and model the dynamic processes at work. In collaboration with the ADRC Neuropathology Core and Sagebio Networks, the Young Lab is using cell lines derived from ADRC research participants to relate participants' genes to their clinical symptoms, as a way to establish general genotype-clinical phenotype patterns across cases of sporadic late-onset Alzheimer's disease. This information will help researchers to categorize IPSC lines and choose risk profiles.
IPSCs are used as tools of discovery to observe mechanisms at cell biology level and perform experiments to identify novel pathways for therapeutic development. So far, Young's research suggests that targeting defects in the cell's endosomal network may be a promising strategy against the disease.
At MODEL-AD, Young presented her lab's work with IPSCs to understand the role of the SORL1 gene in the risk of late-onset Alzheimer's disease. In a new IPSC line, they used CRISPR genetic manipulation to knock out the function of the the SORL1 gene. They are now using this model testing their hypotheses that the loss of SORL1 will impair the endosomal network, and that enhancing SORL1 function provides a therapeutic effect. Ongoing work shows promising results. Young's lab has found that the loss of SORL1 function indeed interferes with the recycling function of the endosomal network, and they observe an increase in amyloid beta in the cells. In contrast, the overexpression of SORL1 in another IPSC model shows a reduction in amyloid beta, a sign of the potential therapeutical value of enhancing SORL1 function.
Read More > Presto Change-O! Neurons in a Dish: A New Approach to Alzheimer’s Disease
Using Worm Models to Search for the Molecular Modifiers of Neurodegeneration
Brian Kraemer, PhD, Research Associate Professor, UW Division of Gerontology & Geriatric Medicine, spoke about the importance of paying attention to the molecular mechanisms of tau pathology, as it is an important protein in sporadic late onset Alzheimer's disease. In fact, the distribution of tau pathology in the brain is strongly correlated with cognitive decline, unlike amyloid beta. He highlighted his lab's work on a candidate gene for tau toxicity, MSUT2, with an eye towards therapeutic potential.
The Kraemer Lab uses the c. elegans worm and mouse models of tau toxicity to discover the role of MSUT2 in controlling or modulating tau. They have found that the overexpression of MSUT2 worsens the behavioral phenotype and increases tau aggregation and neurodegeneration in their models. Conversely, the complete loss of MSUT2 suppresses tau, which ameliorates the behavioral phenotype, tau aggregation, and neurodegeneration. They have concluded that MSUT2 loss of function is protective against tau pathology, at least in animal models.
The perfect drug, in Kraemer's view, would eliminate the action of MSUT2. The team has identified a number of genes, particularly variants of MSUT2, that are strongly protective against severe neurodegeneration in animal models. Kraemer thinks that the perfect drug would emulate the protective effect of these MSUT2 gene variants.
"I think the field needs to identify protective variants to nominate new therapeutic targets," said Kraemer. "This idea comes from the field of cardiovascular disease, which has shown that loss-of-function gene variants that are protective can be rapidly translated into new therapeutic targets in drugs".
There is much to learn about the role of MSUT2, and its biological partners, in the workings of normal and abnormal tau protein in humans. Ultimately, Kraemer envisions targeting MSUT2 or a related risk gene by small molecule MSUT2 inhibitors.
Read more > In a Worm, the Kraemer Lab Studies the Early Signs of Brain Disease
Molecular Staging of Alzheimer's Disease through Lineage Analysis
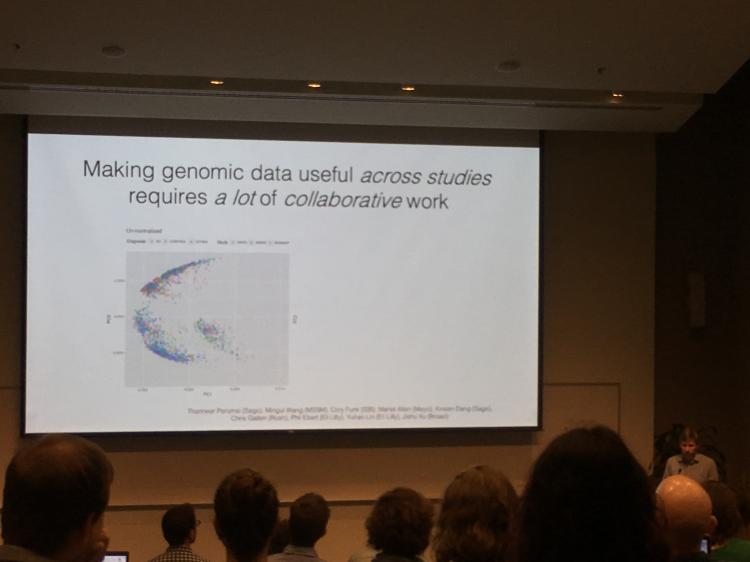
Ben Logsdon, PhD, Director of Neurodegenerative Disease Research at Sagebio Networks and affiliate member of the ADRC, spoke about his work in collaboration with Jackson Laboratories, UW ADRC, and AMP-AD, an initiative of the Accelerating Medicines Partnership. His team analyzes the trove of longitudinal cognitive, neuropathological, and genome-wide gene expression data from multiple cohorts of patients with Alzheimer's disease and without.
"These human cohorts represent one of the opportunities we have to understand the early stages of disease and mechanisms of resilience," said Logsdon. However, the research problem is that each of the study participants and normal controls in these cohorts were, at autopsy, at a different stage of disease or different path to development of disease or continued resilience. Having measures from a person at only one point in time limits what researchers can learn about the drivers of a complex progressive disease. What they really want is to understand the actual process of disease development in the human brain over time.
Logsdon's team used an approach in machine learning called 'manifold learning', which allows a researcher to take an enormous amount of data points and project them into a complex spatial pattern. This allowed them to place each participant on a trajectory, based on data about the levels of RNA expression gleaned from brain tissue.
The knowledge from this modeling will ultimately help researchers to create subgroups of patients based on molecular signatures, come up with model systems that predict an individual's disease trajectory, and create precision medicine treatments.
After finding success in their machine learning approach, the team performed further model analyses to identify the gene pathways that were enriched in the cases predicted to be on the path to developing Alzheimer's disease symptoms. They found pathway enrichments in regulation of apoptosis; and upregulation of splicing, protein stabilization, and regulation of amyloid clearance. "We show that you can have a burden of proof that mechanism are biologically relevant and that there is concordance with estimates of disease staging based on known hallmarks of disease," said Logsdon. •
__
For more information about the mouse models, data, and experimental resources, or training opportunities available through MODEL-AD, visit Model-ad.org.
-Genevieve Wanucha