By Franklin Faust and Genevieve Wanucha
It was one of the longest distances that Thomas Bird, MD ever traveled for science—an eight-hour flight from Seattle to Amarillo, Texas, and a two-hour drive north into the Oklahoma panhandle to arrive at a small church in the small town of Beaver. It was 1987, which he still remembers because those two states were celebrating the 200th anniversary of the signing of the Constitution. He had come for the reunion of a large family he had come to know through phone calls to the UW Alzheimer’s Disease Research Center and a previous visit.
The Reiswig family was unique, in a medical sense—at least twenty members had developed dementia over four generations, with ages of onset from 40 to 75 years old. This family had been described in 1979 by Robert Cook-Deegan, MD at University of Colorado, but their particular shared ethnic background had gone unrecognized. In the late 1980’s, researchers were just beginning to understand that some cases of dementia linked to Alzheimer’s disease likely had a genetic cause. While Bird knew that inherited or 'familial' Alzheimer’s disease only accounts for a tiny fraction of cases, he also knew studying these unique families could open the door to better understanding the mechanisms of disease and, possibly, a treatment target for all cases.

A church in Beaver, OK. Source: Blog Oklahoma
That day, seated in the church pews, the Reiswig family listened as Bird explained the details of their neurogenetic study. The family signed consent forms to participate, and Bird took blood samples, which he sent back to the team’s research nurse, Ellen Nemens (now Ellen Steinbart). The team would extract the blood cells’ DNA and search it for what could be a faulty genetic factor. Importantly, the family shared their history so the researchers could create a pedigree, a family health history indicating relationships and disease cases using universal symbols.
Bird brought over twenty years of experience working with families with genetic disorders, such as Huntington’s disease, at the UW Medical Genetics clinic, which he founded in 1974. Medical geneticist Ephrat Levy-Lahad, MD, who would later bring this family study to fruition in 1995, remembers: “Tom works so closely to the families that he sees as a physician, and while he's not a molecular biologist, he has that genetic vision – seeing families and seeing patients and thinking about problems in a genetic way. I really learned to take that approach at the ADRC, and I still practice it. The families and researchers share a goal in that kind of project, and that is very powerful to me to this day.”
Bird knew that at-risk families often have a person who is particularly motivated to address the problem and seek help, a firebrand whose enthusiasm will inspire even hesitant relatives to open up about painful topics and organize them to join a research effort.
This family was no different. Ester May Reiswig was eager to help shed light on the disease that had recently taken her husband. “Thanks to her, the family reached out to us,” says Bird. “They were concerned that they had hereditary Alzheimer’s disease and that nobody was paying attention to it.” Bird and Nemens fostered a dialogue. He listened to her as she spoke about her husband’s ancestors and genealogy.
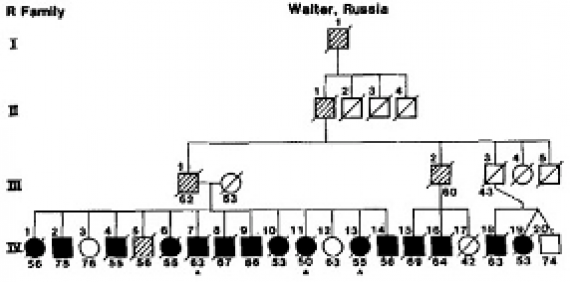
Part of the pedigree of the Reiswig family showing multiple persons over four generations affected by Alzheimer's. Courtesy, Thomas Bird
Reiswig’s late husband’s family was part of an ethnic group called the ‘Volga Germans’, who had moved from the Hesse region of Germany to the Volga River region in Russia at the behest of Catherine the Great in the 1760s. Later, amidst the political and economic tumult of the 20th century, many came to the US to settle in small villages along the trans-continental railroad. And the people busy catching up at the reunion weren’t the only ones of Volga German ancestry with a history of Alzheimer’s disease, she shared. Reiswig knew of two other families who had been documented as suspected genetic Alzheimer’s cases, but no one had asked them about their background—as Bird was accustomed to do.
“As I was sitting there listening to her story, I remembered that one of our families back in Seattle had a German last name, and a relative who wrote a book called The Volga Pilgrims,” says Bird. “It got me thinking.” Bird continued to listen as Reiswig recalled that these affected Volga German families came from the same two tiny villages in southwestern Russia, Frank and Walter. “I realized that there could be many families whose ancestors migrated over from those same villages—distant relatives with a gene for Alzheimer's,” Bird said. He quickly checked the families already in the ADRC study, discovering three more families with a Volga German connection.
Over thirty years later, Bird would recall what he calls his “Eureka Moment:” The Volga Germans would be a particularly useful collection of families in the search for an Alzheimer’s disease gene because the affected family members would almost certainly share the same responsible gene; even more significant was that these family members could all be related to a common ancestor who first introduced the mutation into the family line. His question: Which gene is it?
“I still remember the day when Tom came to my office really excited one morning, and he started telling me all about how he had visited this family and learned that they were Germans from Russia,” says Gerard Schellenberg, PhD, now Professor of Pathology and Laboratory Medicine at University of Pennsylvania and Director of the Penn Neurodegeneration Genomics Center.
Nemens’ degree in German became a valuable asset. She started digging through the ADRC Registry, which is a large pool of potential research participants who agree to be contacted for research. She kept an eye out for families with German last names and contacted them to find out their place of origin or family’s history. The fact that Nemens could pronounce the names of German villages surprised and delighted the families. By 1990, seven families of Volga German ancestry, all originating from the same two villages in Russia, made up one quarter of the families being studied by the ADRC team in the Center’s first genetics project.
The vast majority of people of Volga German descent do not carry this genetic factor and do not necessarily live at a higher risk of dementia than the general population. Take for example Pacific Northwest natives Arlene and Al Noreen, who are known to many as active and cognitively sharp “super-agers” in their mid 90s. Arlene’s own grandparents immigrated with their family from the Russian village Walter on the Volga River to escape civil unrest. By the time the family reached the United States in 1901, the group included an infant who would one day become Arlene’s mother. They settled in Walla Walla, Washington State, close to other Volga German immigrant families.

Alfred and Arlene Noreen in 2019
Arlene, age 96, attributes her enduring health to several key factors now recognized to boost brain health and lower dementia risk. She has lived a life full of cognitive stimulation from a childhood spent playing in nature and learning in primary school, libraries, and then college at the University of Washington. Raising five children, volunteering in classrooms and museums, and serving as a mountain tour guide with her husband Al, Arlene has constantly embraced an intensely active and social life. But most importantly, she reports, the real secret to their health must have a lot to do with how they have supported each other in a difficult task: “We follow the doctor’s orders.”
Ever since Al, now age 95, developed high blood pressure in his 40s while working as engineer at Boeing in Seattle, the couple has eaten a very low salt diet and lots of fresh vegetables (though they still enjoy Arlene’s grandmother’s family recipes for sauerkraut with apple slices and beef-stuffed cabbage buns). Al still swims in the community pool for 45 minutes every morning and volunteers in a local botanical garden.
Arlene, who has helped to take the residents of a nearby memory loss facility on walks, says her heart goes out to the families of Volga German ancestry who were affected by Alzheimer’s disease, through simple bad luck. She sees these individuals who share her ancestral immigration story not as victims who succumbed to Alzheimer’s disease, but rather as brave individuals who helped researchers form a new framework of brain disease that considered risk as well as resilience.
The Foundational Theme of the ADRC
Several years prior, in 1983, this ambitious project on familial Alzheimer’s was a moonshot proposal. This longitudinal study aimed to follow research participants over time, collect and store biospecimens, and use emerging technologies in genetic analysis. The effort would require sustained, long-term funding to have a shot at success. Fortunately, the National Institute on Aging had recently issued a new federal initiative that called on universities to apply to become Alzheimer’s Disease Research Centers (ADRCs). The NIA aimed to create many ADRC’s around the county, which would each have different specialties and themes to foster the study of Alzheimer’s disease from unique areas of expertise and institutional strengths. To this day, the ADRCs aim to provide a continuous infrastructure and necessary resources to support Alzheimer’s and neurodegenerative research, and the training of young scientists.

Bird soon learned that the plans for an ADRC at the UW were already falling into place, thanks to George Martin, MD, then a Professor in the UW Department of Pathology who was driven by an intense interest in the genetics of neurological diseases. Now age 93, Martin clearly remembers sparking the formation of the ADRC. Martin quickly garnered enthusiastic support from the UW School of Medicine Dean’s office. He started to form the team who would carry out the foundational project, and Bird quickly signed on to lead the project in the genetics of familial Alzheimer’s disease.
“To build a good team, we started by looking for someone who was “hands-on with genetics,” says Martin. “I went to the late Dr. Ben Hall, a brilliant yeast geneticist and an old friend, and—actually—a talented gardener. I remember I used to go to his house to get fresh corn that he grew as a hobby.” Hall, in turn, recommended a post-doc named Gerard “Jerry” Schellenberg. He had received mentoring from Clem Furlong, PhD, UW Professor of Medical Genetics, an expert in gene-environment interactions.
“This is a guy who is now world famous in the Alzheimer's field, but in 1983, he didn't know what it was,” says Bird of Schellenberg. “I told him we needed somebody to isolate DNA and look at DNA markers in this little-known disease.” As it happened, Schellenberg was itching for a new challenge. He agreed to run the new ADRC Molecular Genetics Lab and develop genotypes—pieces of an individual’s full genetic profile obtained through the use of biomolecular tools at specific points in human DNA called genetic markers—represented in a chart with the numerical language that computers can understand.
“Bird knew I was getting interested in human genetics,” says Schellenberg. “I'd done bacterial genetics, but I'm interested in human genetics and the human gene mapping. He just came up to me and said, 'Well, we're going to put in this application to the NIA. Are you interested in working on this?'And I said, 'Fabulous.' I cared more about genetics than Alzheimer's specifically, and now it's probably the opposite way around; I care more about Alzheimer's than anything else. It was an opportunity to do what I really wanted to do.”
NIA reviewers traveled across the country to hear and evaluate the UW’s proposal in person. But when the initial site visit team arrived on campus in 1983, Martin was shocked. “There was not a single geneticist or cell biologist, which was very surprising, given the specific aims [of our project]” says Martin. “The site visitors, mostly geriatric psychiatrists, were very skeptical about the idea that there were major gene mutations responsible for Alzheimer's disease. We were presenting a plan for genetics, cell biology, and epidemiology, and they were puzzled as to why we would want to emphasize genetics in our Center because it was not considered a genetic disease. They thought we were out of our mind, and they did not recommend funding.”
The UW team only became more motivated to show the NIA that genetic family studies held the key for understanding the mechanisms of Alzheimer’s disease. The team persisted and prepared to shoot for a spot among the remaining five center allocations coming up the following year. Meanwhile, Martin impressed upon the NIA Program Manager the importance of including application reviewers who had an interest in genetics or biology.
Building the Case for the UW ADRC

Thomas Bird examining historical Volga German family records, 2020.
It was Bird who built a better case for their Center’s funding, following a research direction that would eventually lead him to the Volga German family a few years later. Families with a history of Alzheimer’s disease continued to be referred to him by Washington State neurologists who were puzzled by their shared predisposition to the disease. “Many of the Alzheimer’s-type dementia families referred to me during this time fortunately happened to have individuals who had undergone an autopsy, which was necessary for an Alzheimer’s diagnosis at the time,” says Bird. “Back then, ‘senility’ was still the normal term – you had to have an autopsy that proved the presence of Alzheimer’s plaques and tangles.” Today, brain imaging technology or spinal fluid tests allow doctors to see this protein evidence in living patients, but a diagnosis of Alzheimer’s disease at the time required an expert look at the brain postmortem. Shuzo Mark Sumi, MD, a neurologist, neuropathologist, and lauded mentor, now Professor Emeritus of UW Neurology and Pathology, started to study the brain tissues from individuals in these families who had consented to brain autopsy when they died. The verified presence of plaques and tangles further strengthened the argument that these cases were truly Alzheimer’s disease.
When the NIA came back for the second round of visits, Bird was prepared to make what he hoped was an air-tight case. The team presented their relatively large cohort of research participants from these unique families, autopsy data from several individuals from these families, as well as their advanced genetic tools and qualified investigators who were ready to take on this large-scale challenge. “I remember saying: ‘These pedigrees show three or four generations. Males and females are both affected. It’s autopsy-confirmed Alzheimer's disease,” recounts Bird. “If that isn't genetic, I don't know what it is.”
“Tom, I think I’d buy a used car from you,” whispered Schellenberg after presentation to the site visitors. This ADRC application, a 537-page compendium of yellowing typewritten sheets, now rests on a shelf on the 14th floor of the Ninth and Jefferson Building at Harborview Medical Center, in the current Director’s office.
“You have to remember that in the 1980s, before the era of computers, the grant applications were huge masses of paperwork,” said Bird. “This big center grant had to be put all together with a fax, copy, and Xerox machines, and the job fell onto the lap of Marie Walters, the first ADRC Program Manager. And she was able to do it. I remember seeing tables with all the paperwork that was necessary for submitting the Center grant. And Walters was able to keep track of all of that really well, and keep the Center funded through the years.”

Marie Walters, 2020
In 1985, under the founding directorship of George Martin, the UW received funding in the amount of $6,697,620 (about $16,000,000 by today’s standards) to become one of the first of ten NIA ADRCs in the country. Each directorship transition over the next 35 years would shift the ADRC’s major research themes and contributions. From Directors George Martin, MD (1985-1999) to Murray Raskind, MD (1999-2012), to Thomas Montine, MD, PhD (2012-2016), to Thomas Grabowski, MD (2016 – present, as of 2020), the Center built historic strengths in genetics and genomics, caregiving programs, fluid biomarkers, and neuropathology, and newer strengths in cell biology, stem cell model technology, genetic profiling of neurons, and brain imaging, along with transformative dementia-friendly community outreach at the UW Memory and Brain Wellness Center.
Through these successive waves of innovation however, the original family genetic research of the ADRC’s original Cores and Projects would stay a constant thread and foundation of future discovery. “The ADRC’s family study was one of the most long standing projects that the ADRC had during my time,” says Marie Walters, “which was unusual, because when an ADRC seeks a renewal of funding for the next 5-year cycle, the NIA evaluates progress, some projects stop, and others start. But, most other Centers weren’t approaching Alzheimer’s from a genetic angle. Our researchers were unique in our expertise in that area, among the first in their fields to study Alzheimer’s in that vein.”
How To Search for a Disease Gene
NIH
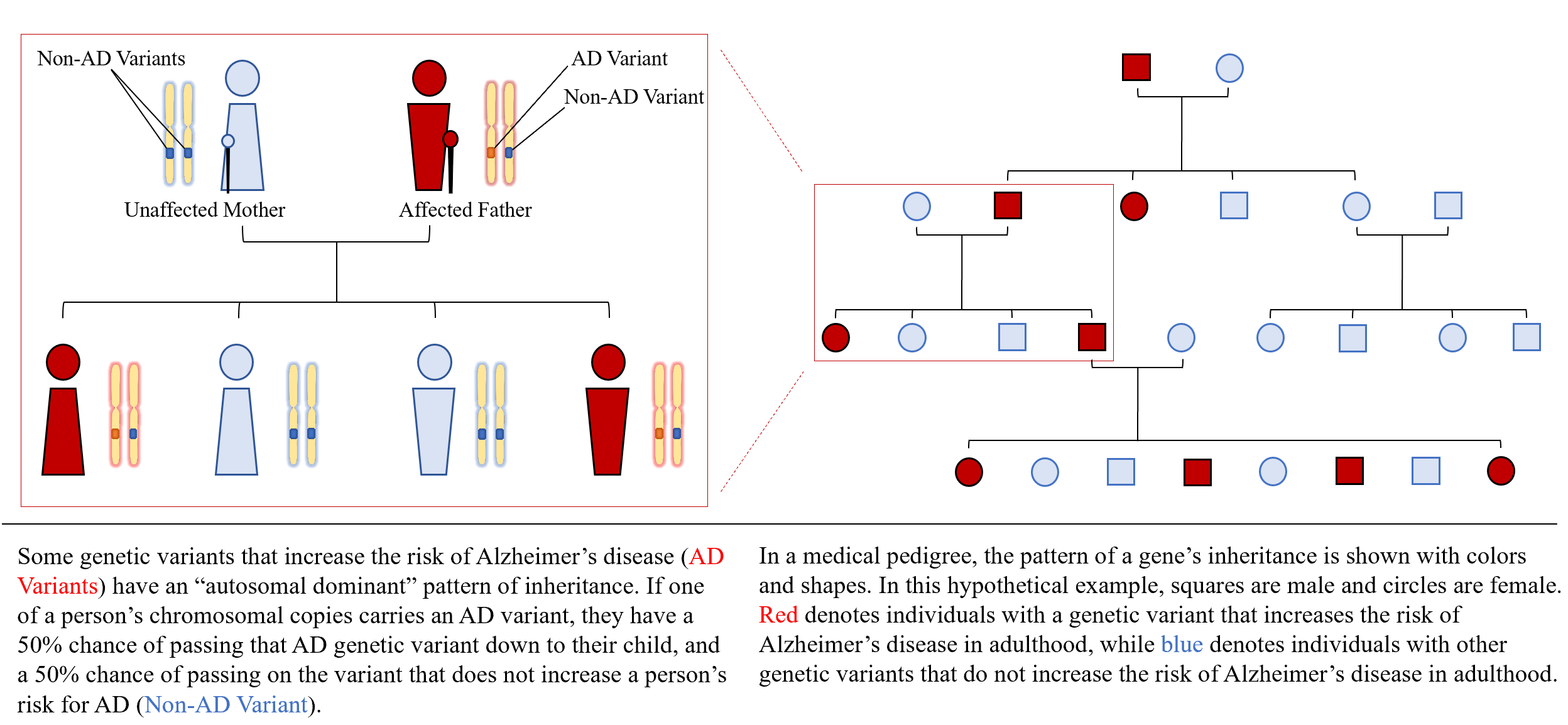
Chromosomes are groupings of the roughly 100,000 human genes, with 23 pairs occurring in every cell in the human body —one chromosome in each pair comes from Mom, and one from Dad. A gene is a unique section of DNA that codes for the production of a specific protein. Copying errors called mutations occur as cells divide, and result in slight variations in the DNA sequence. These mutations can result slightly different versions of the protein that a gene codes for. Most of the time, these slight variations are of little or no consequence to human health—like a word in a long sentence being replaced by a synonym. However, sometimes, the mutation may affect the meaning of the whole sentence—this can provide an advantage to survival, or a disadvantage (a disease). Unless the mutation has a severe effect in early life, it will have a 50% chance of being passed down to the next generation.
Currently, single gene mutations are known to cause an estimated 3% of Alzheimer's cases and 10% of cases of Parkinson's disease, amyotrophic lateral sclerosis, and frontotemporal degeneration. These ‘familial’ cases often follow patterns of ‘autosomal dominant’ inheritance, meaning that if a person carries the mutation, then there is a 50% chance they will pass it on to their child.
To study a familial neurodegenerative disease, such as those affecting ADRC families, researchers first need to identify the disease-causing gene. In the 1980s, the first step was to track down which of the 23 chromosomes the gene lived on. “Once the chromosome is revealed, the next step is to locate the actual gene, and then learn what process in the brain that gene is controlling. When we understand what process the gene is controlling, we may be able to alter the process or prevent it from occurring," Bird described in 1989. "That's clearly the long-term goal of genetic research."
Fifteen years before that statement, Bird had used the knowledge of genetic inheritance to search for the location of a disease-causing gene in the human genome. Shortly after Bird’s founding of the first neurogenetics clinic at UW, he began searching for a mutated sequence of DNA in one of his first families of patients – a family with an unusually high occurrence of a rare neurogenetic condition called Charcot-Marie-Tooth (CMT) disease.
Bird started from a family pedigree—a map of the family tree that shows multiple generations of descendants starting from the earliest known relative and that denotes the presence or absence of the disease being researched (see Explainer). Through colors and symbols, it gives an overview of which family members had the disease, and which family members were spared. The pattern of inheritance looked autosomal dominant. To trace the disease gene down to a specific chromosome, he needed some sort of genetic marker—a piece of DNA whose known position can be compared to the inheritance of a disease.
At the time, a handful of genes that determined certain blood group types had been linked to specific chromosomes. Bird noted the family members’ blood group types on the pedigree. To his surprise, he found that a blood group called “Duffy” was always positive in family members affected by CMT and negative in unaffected members. The presence of the Duffy gene strongly correlated with the presence of whatever gene was causing CMT disease in this family.
The two genes appeared to be ‘traveling together’ from generation to generation. Bird knew that genes situated close to each other on the same chromosome are more likely to stay together through generations of chromosomal “cross-over”—an event preceding a sperm or egg’s formation. In cross over, each pair of chromosomes swap sections of DNA with each other before one of the chromosomes in the pair goes into an egg or sperm. So, the chromosomes that go into the sperm or egg cells contain a little bit of DNA from both of the grandparents. The likelihood that cross-over will split up genes onto separate chromosomes depends on the genes’ physical distances to each other. Therefore, in Bird’s research case, it was likely that the Duffy gene and the mystery disease variant were located very close together, enabling the two genes to be passed down together for generations.
Fortunately, Bird had gotten lucky with Duffy. “When the Duffy blood group is positive, it actually puts a kink in chromosome 1, and you can see it under a microscope,” says Bird. “Because the two genes were traveling together so closely, we knew that the Charcot-Marie-Tooth disease gene had to be on chromosome 1 too.” Bird’s research took place before researchers knew the chromosomal “address” for many genes, so his successful linkage of a disease gene to a chromosome was fairly serendipitous. “Back then, if you didn't happen to have a marker for the location of the gene, you were out of luck,” says Schellenberg. This success made Bird a candidate for leading the ADRC’s first familial genetics project.
By the time the ADRC launched in 1985, researchers had access to a more advanced method for tracking down a disease gene thanks to genetic advances of the Human Genome Project. “Brand-new research showed that we could take human DNA, chop it up into little chunks, and use them as markers for linkage studies,” says Bird. Those markers gave scientists genomic “addresses” for genes on every chromosome. With known locations for so many genes, scientists started using ‘genetic linkage analysis,’ a powerful new statistical method to detect the chromosomal location of disease gene, based on the understanding of genetic inheritance that Bird had used in the 1970s.
EXPLAINER: AUTOSOMAL DOMINANT INHERITANCE OF ALZHEIMER'S DISEASE (Click on Image to View)