November 26, 2014
UW researchers uncover the molecular basis of the heartbeat
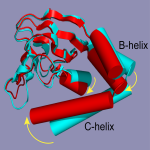 Assistant Professor Stefan Stoll (Chemistry), Professor William Zagotta (Physiology & Biophysics), and co-workers have used double electron-electron resonance (DEER) spectroscopy to determine the structural origins of the regulatory function of cyclic adenosine monophosphate (cAMP) on an important ion channel. Their work reveals that binding of the cAMP induces a large structural change in the intracellular part of the channel. The ion channel studied, a hyperpolarization-activated cyclic nucleotide-gated (HCN) ion channel, is critical to the function of heart, as it is part of the heart’s natural pacemaker. The HCN channel is crucial in regulating the heartbeat: binding of cAMP to HCN increases of the heart rate. This work, reported in the Proceedings of the National Academy of Sciences, could form the basis for better drug design for disorders of electrical signaling in the heart. (A movie showing a model of the structural change can be downloaded in Quicktime format from: http://felix.chem.washington.edu/HCN_DEER_movie.mov.)
Assistant Professor Stefan Stoll (Chemistry), Professor William Zagotta (Physiology & Biophysics), and co-workers have used double electron-electron resonance (DEER) spectroscopy to determine the structural origins of the regulatory function of cyclic adenosine monophosphate (cAMP) on an important ion channel. Their work reveals that binding of the cAMP induces a large structural change in the intracellular part of the channel. The ion channel studied, a hyperpolarization-activated cyclic nucleotide-gated (HCN) ion channel, is critical to the function of heart, as it is part of the heart’s natural pacemaker. The HCN channel is crucial in regulating the heartbeat: binding of cAMP to HCN increases of the heart rate. This work, reported in the Proceedings of the National Academy of Sciences, could form the basis for better drug design for disorders of electrical signaling in the heart. (A movie showing a model of the structural change can be downloaded in Quicktime format from: http://felix.chem.washington.edu/HCN_DEER_movie.mov.)
To learn more about Professor Stoll and his research, please visit his faculty page and research group website.
To learn more about Professor Zagotta and his research, please visit his faculty page.